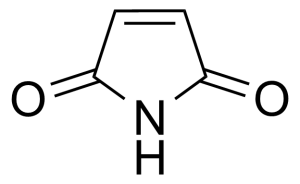
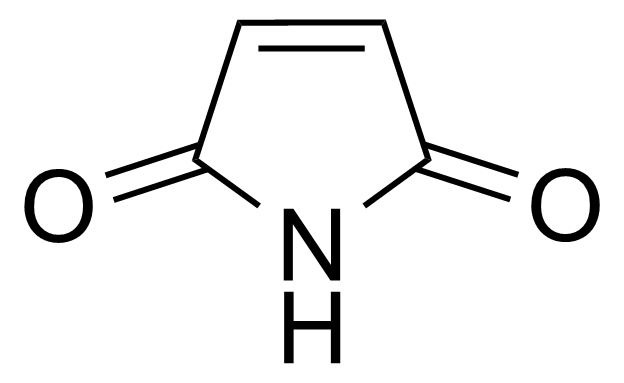
Maleimide
3-pyrroline-2,5-dion ; 2,5-Pyrroledione ; 2,5-Dioxo-3-pyrroline ; 1H-Pyrrole-2,5-dione ; 2,5-dihydro-1H-pyrrole-2,5-dione ; Maleic imide
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information
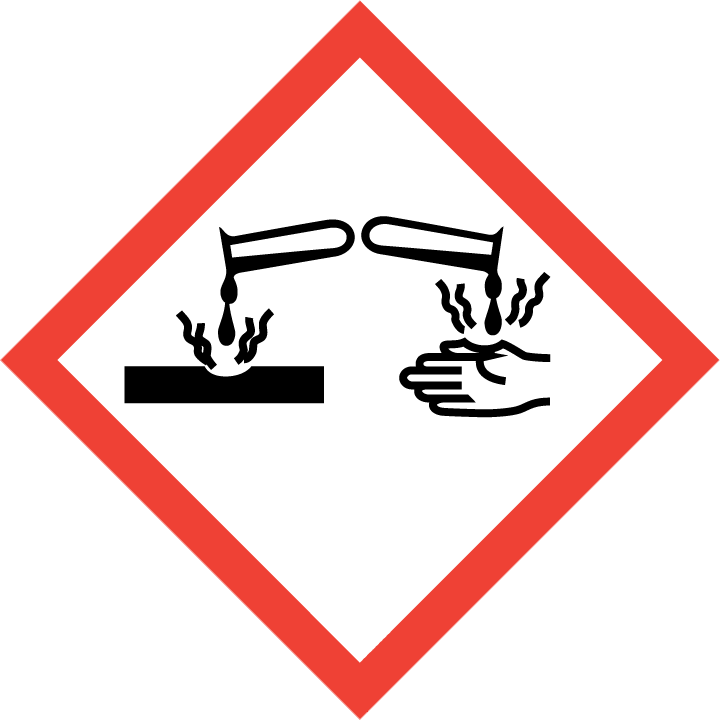

H301 – Toxic if swallowed
H312 – Harmful in contact with skin
H314 – Causes severe skin burns and eye damage
H317 – May cause an allergic skin reaction
H318 – Causes serious eye damage
H332 – Harmful if inhaled
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P310 – Immediately call a POISON CENTER or doctor/physician:
P301+310 – IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P301+330+331 – IF SWALLOWED: Rinse mouth. Do NOT induce vomiting
P302+352 – IF ON SKIN: Wash with soap and water
P304+340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Maleimide is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Maleimide in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Maleimide is versatile building block in organic synthesis. A special feature of the reactivity…
Show full descriptionGeneral description and preparation of Maleimide:
Maleimide [541-59-3] or 2,5-Pyrroledione is a unsaturated imide which name is a contraction of maleic acid and imide. It is a white crystalline solid with the melting point of 92-93 °C.[1] Maleimide can be synthesized via thermal decomposition of N-carbamoylmaleimide formed by reaction of maleic anhydride with urea.[2] In general, maleimides are produced by ring-closure imidation of various maleinamic acids in an organic solvent capable of forming an azeotrope with water in the presence of an acid catalyst and metal-containing compound (zinc acetate) as a promoter.[3]Application of Maleimide:
Maleimide is versatile building block in organic synthesis. A special feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by Michael additions or via Diels-Alder reactions. Natural maleimide derivatives (ferinomalein, showdomycin, pencolide, turrapubesin) with the promising biological activities were isolated from bacteria and fungi.[4] Recently, maleimide, N-ethylmaleimide, N-methylmaleimide and N-phenylmaleimide have attracted the interest due to the cytotoxicity toward tumor cell lines through the inhibition of human topoisomerase II.[5] Maleimide-mediated methodologies are among the most used in bioconjugation.[6] Maleimide-functionalised polymers and liposomes exhibit enhanced ability to adhere to mucosal surfaces (mucoadhesion) due to the reactions with thiol-containing mucins.[7]Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| Acetohydroxamic acid | 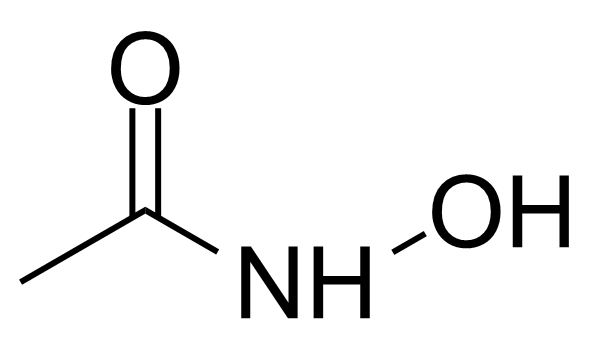 | [546-88-3] | GEO-00010 | |
| 1-Adamantanecarboxylic acid | 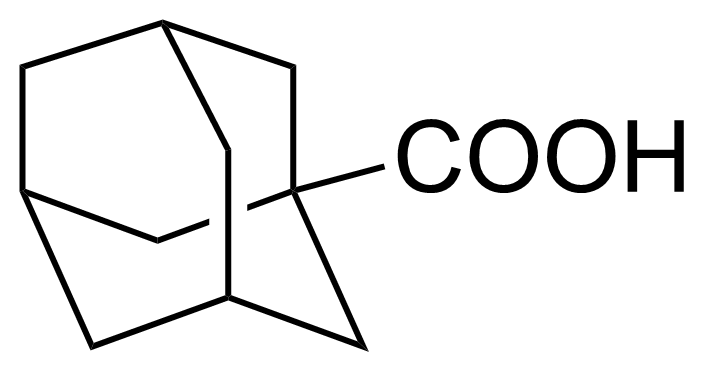 | [828-51-3] | GEO-04336 | |
| New | 4-Amino-5-nitrophthalic acid | 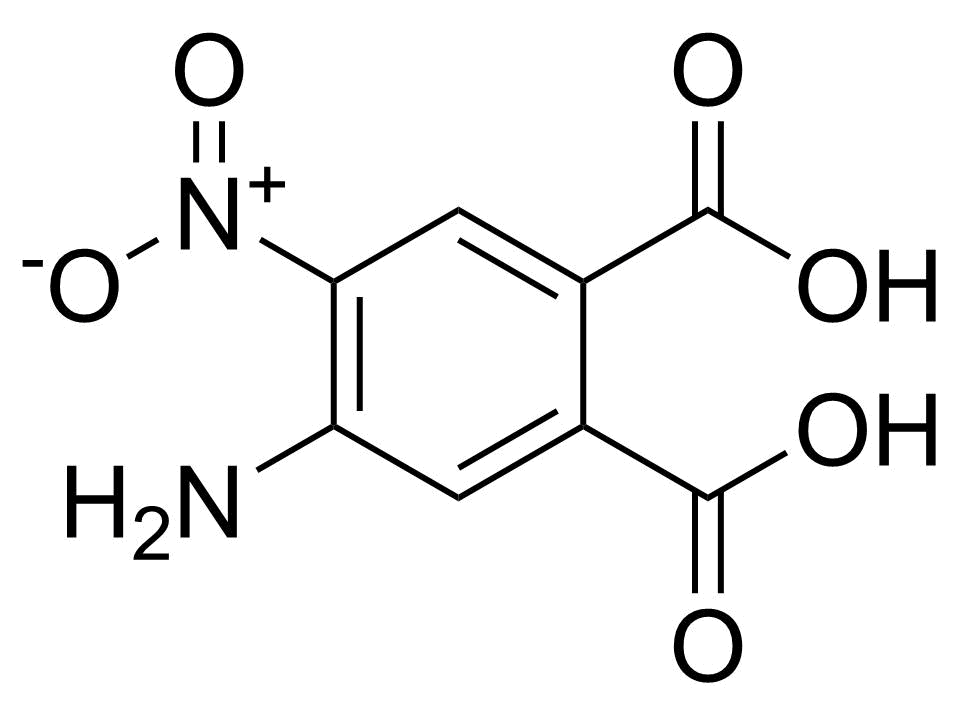 | [89939-49-1] | GEO-04810 |
| 3H-1,2-Benzodithiol-3-one 1,1-dioxide | 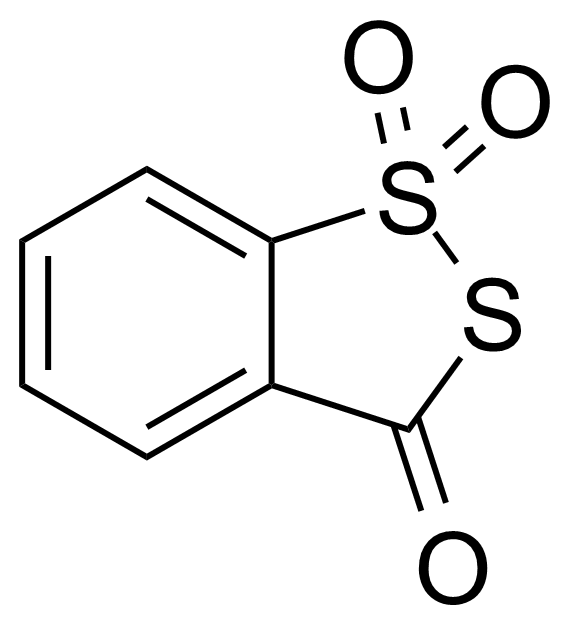 | [66304-01-6] | GEO-04361 | |
| Benzo[b]thiophene-2-carboxylic hydrazide | ![Structure of Benzo[b]thiophene-2-carboxylic hydrazide](https://georganics.sk/wp-content/uploads/2021/05/GEO-00294_Benzobthiophene-2-carboxylic_hydrazide.png) | [175135-07-6] | GEO-00294 | |
| N-Benzylmaleimide dihydrate | 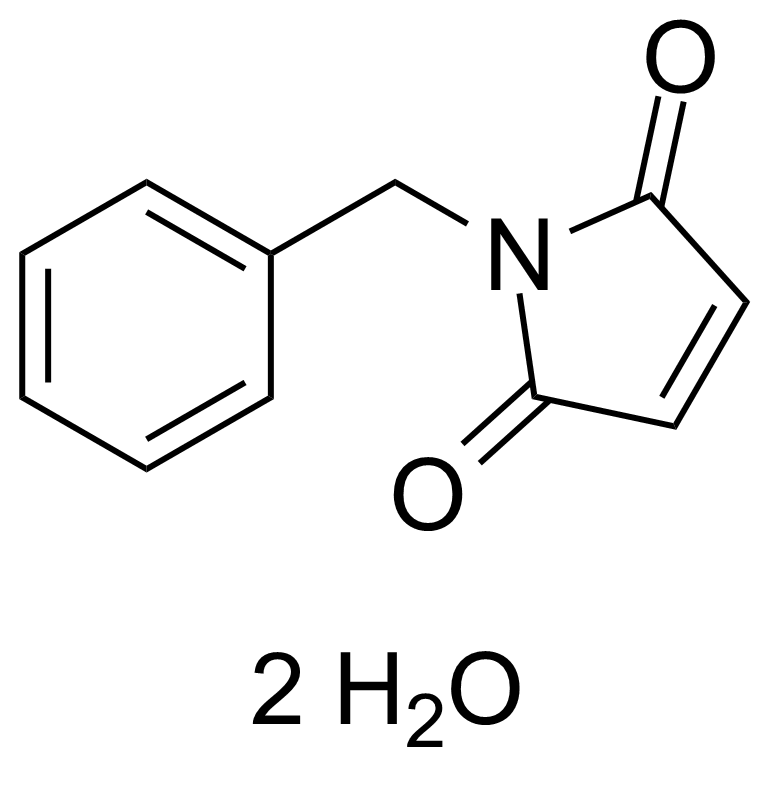 | [1631-26-1] | GEO-00303 | |
| Bromoacetic acid anhydride | 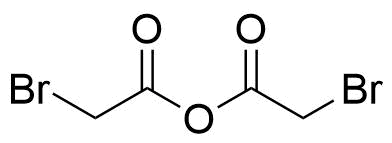 | [13094-51-4] | GEO-04577 | |
| 2-(6-Bromohexyl)isoindoline-1,3-dione | 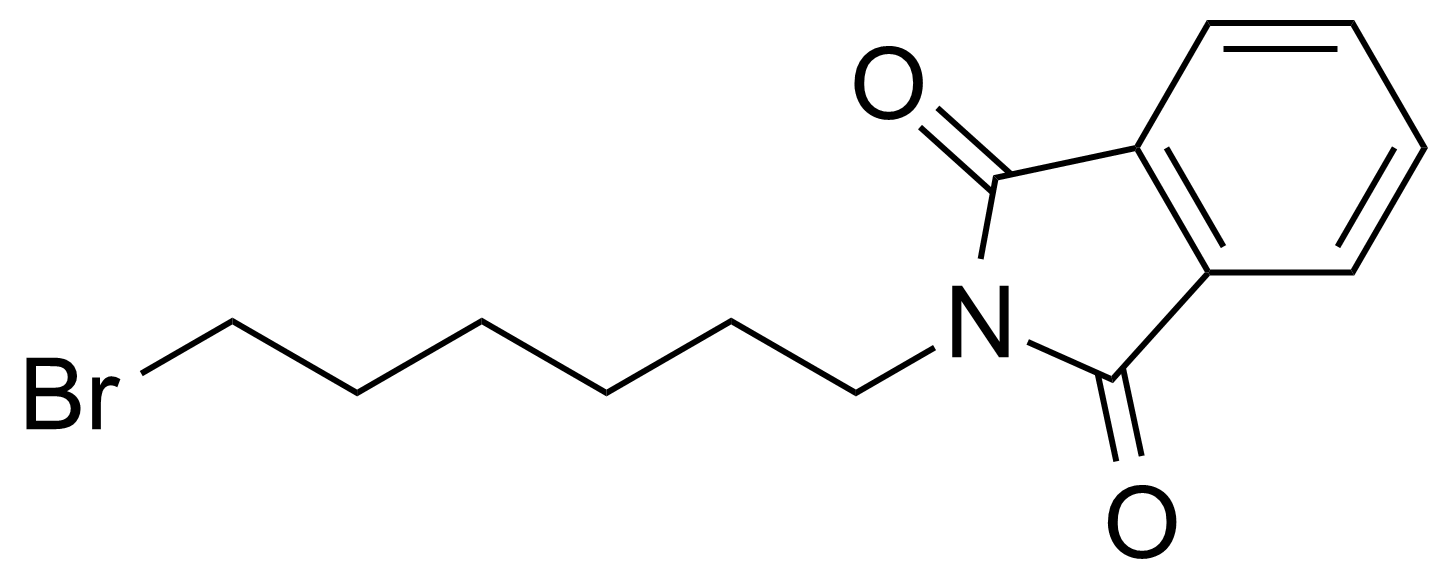 | [24566-79-8] | GEO-04399 | |
| New | 7-Bromoisoquinoline-4-carboxylic acid | 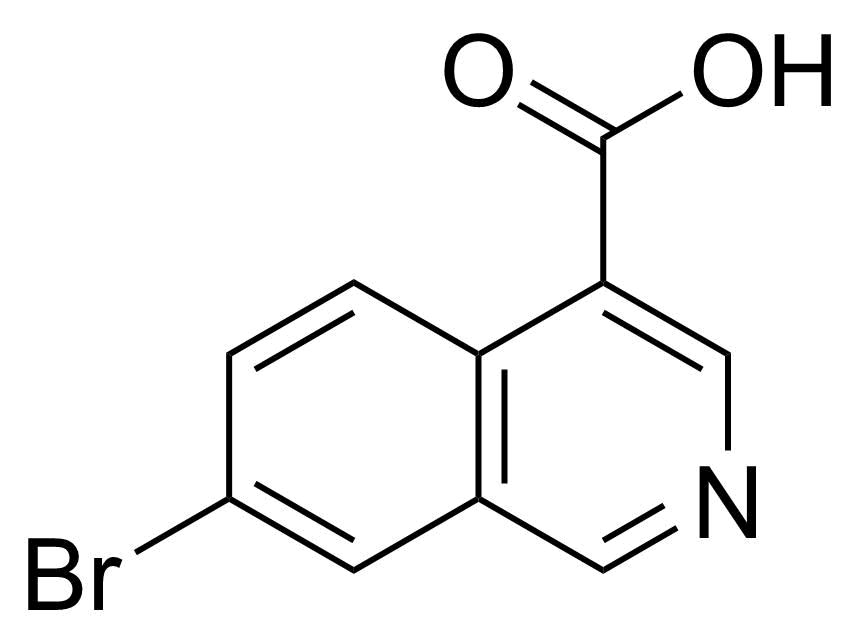 | [31009-04-8] | GEO-04828 |
| Bromomaleic anhydride | 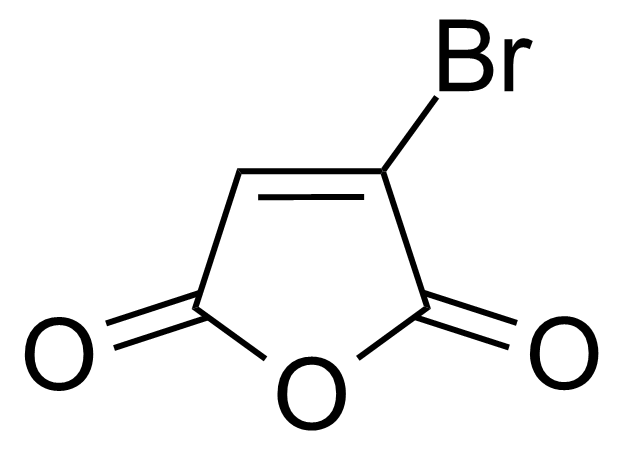 | [5926-51-2] | GEO-00484 |