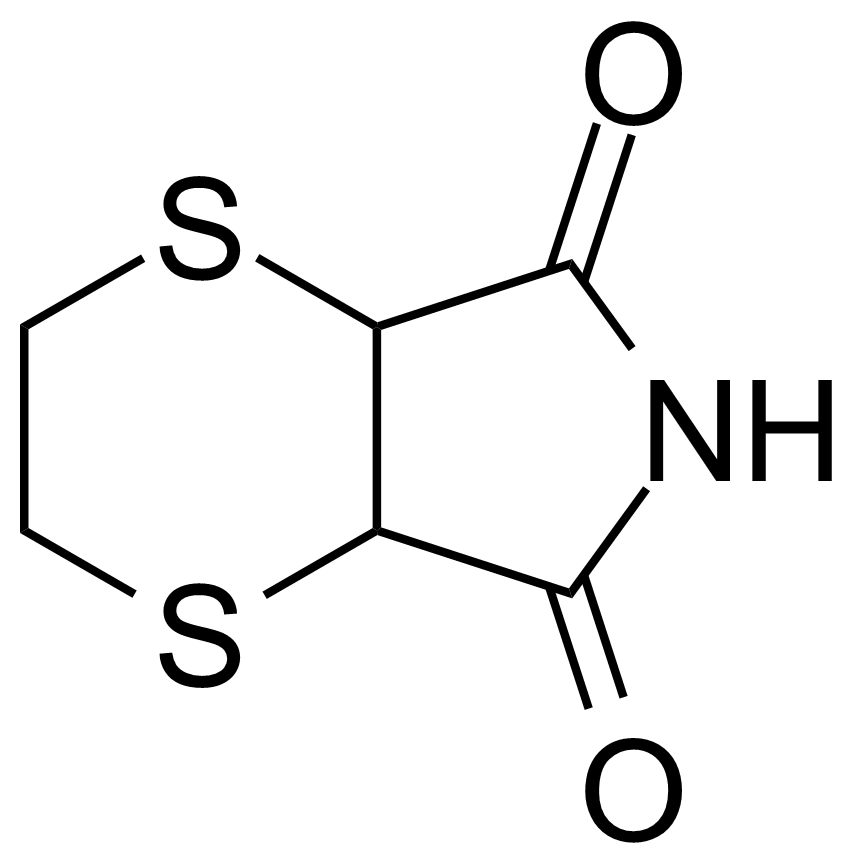
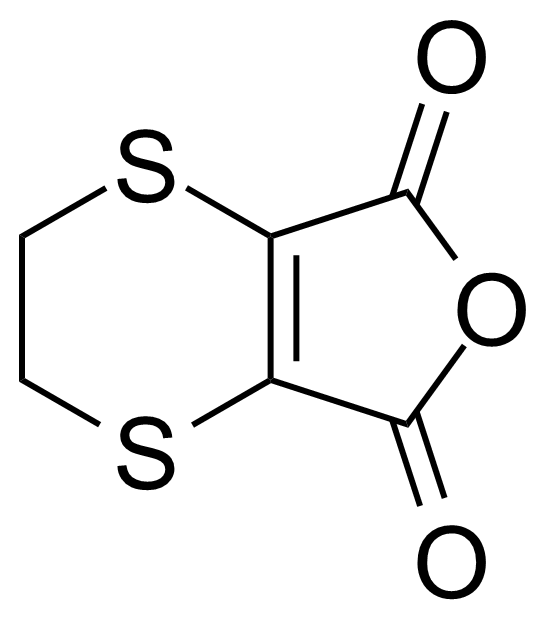
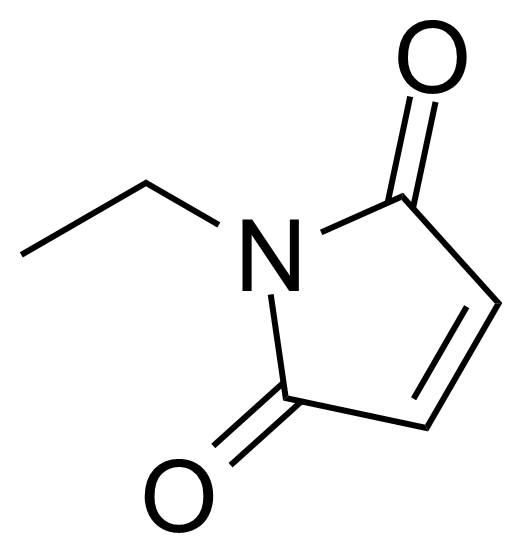
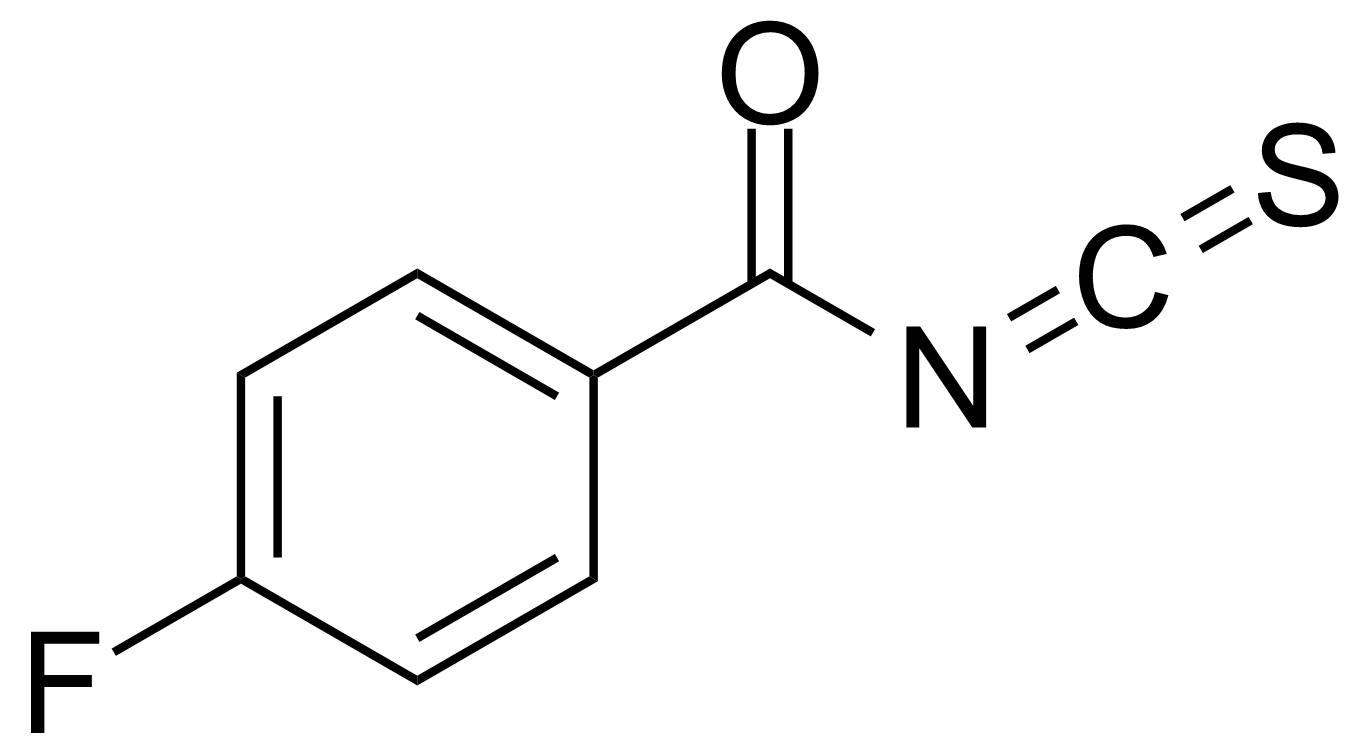
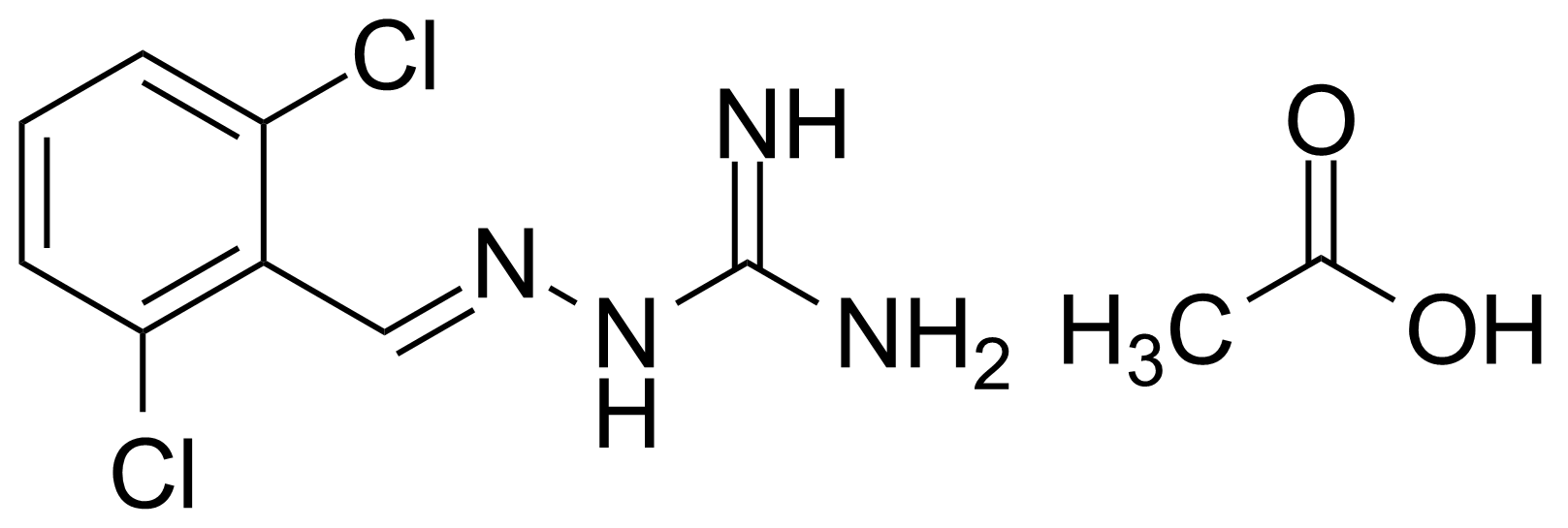
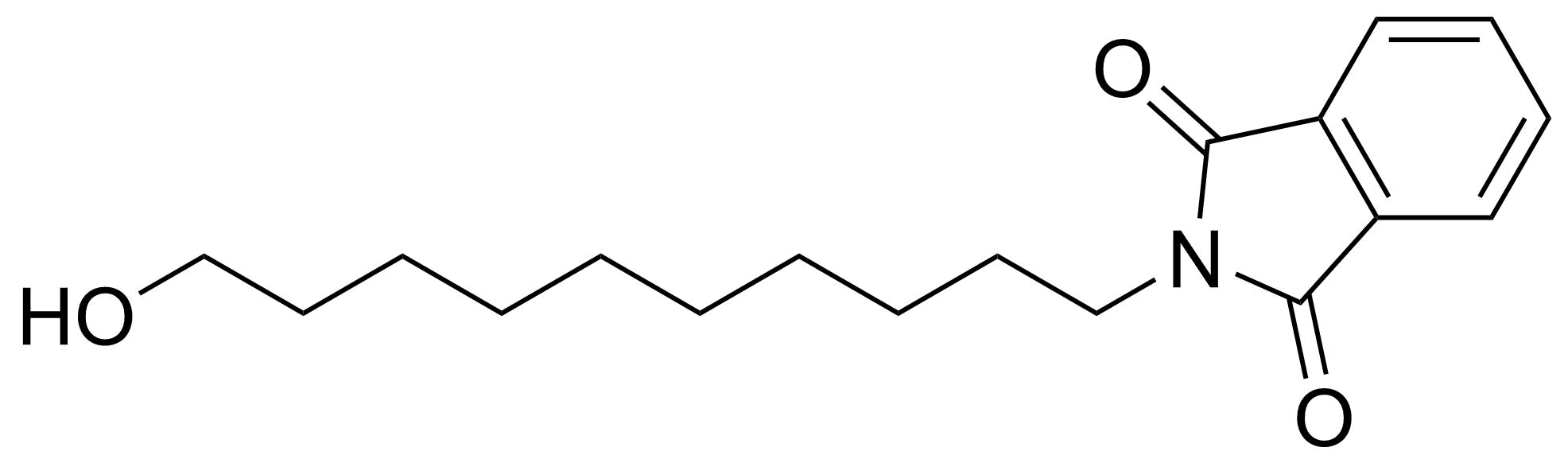
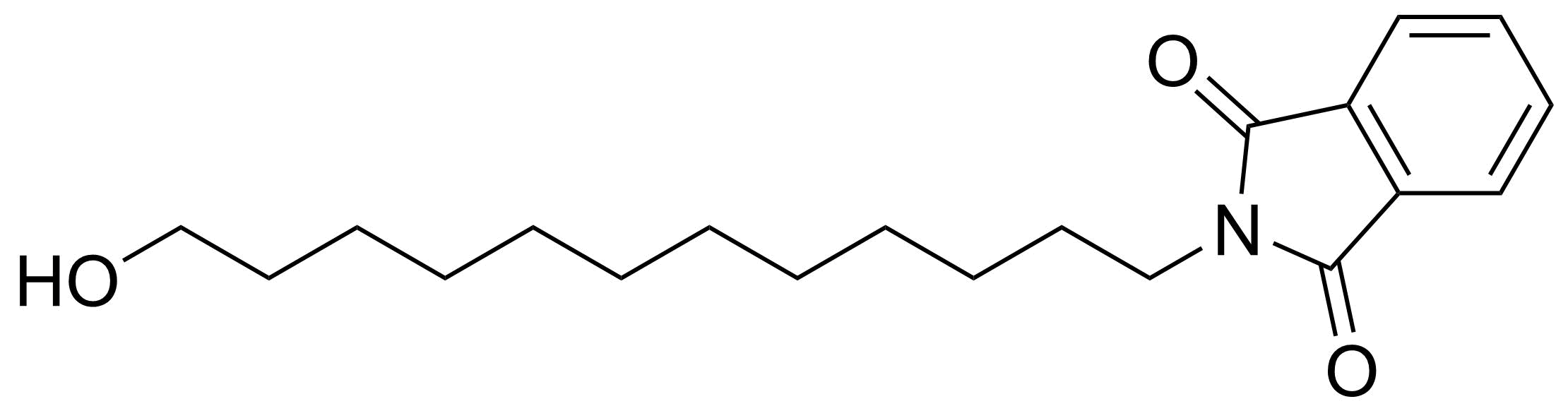
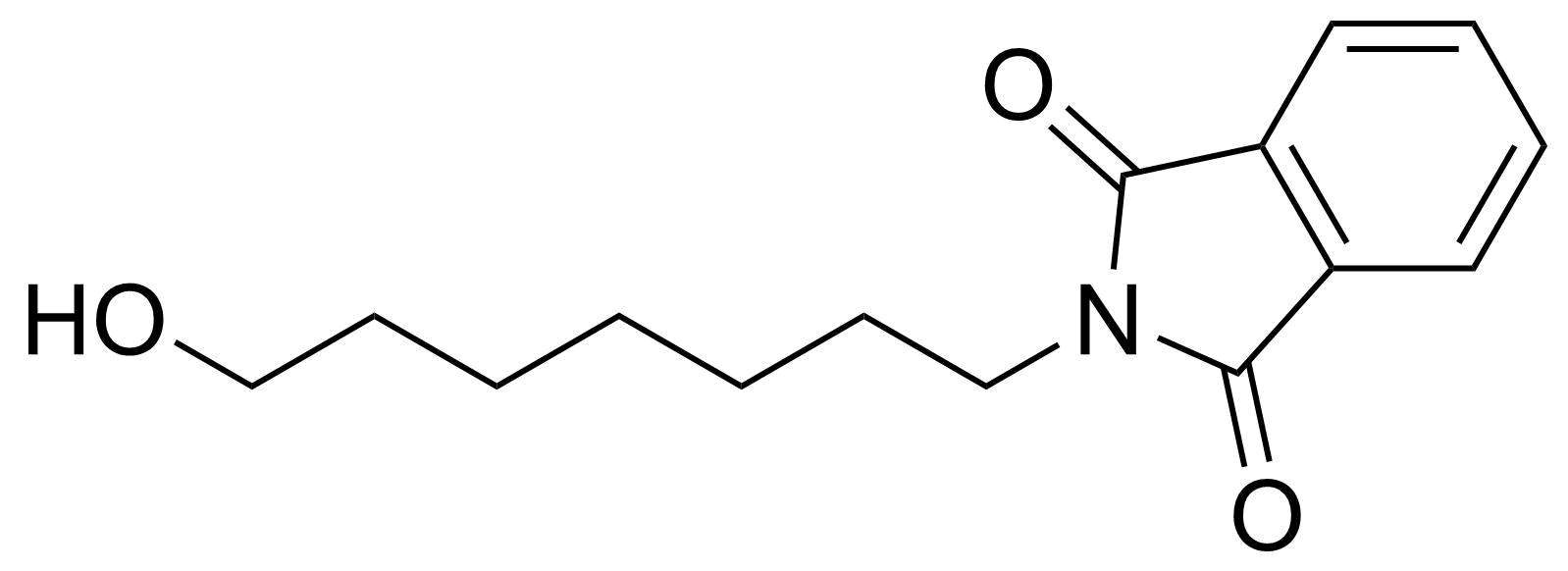
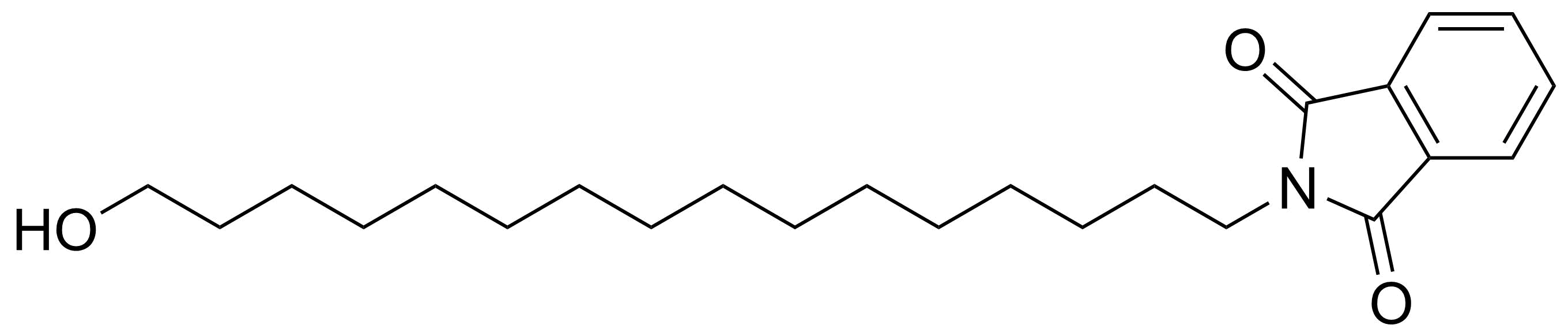
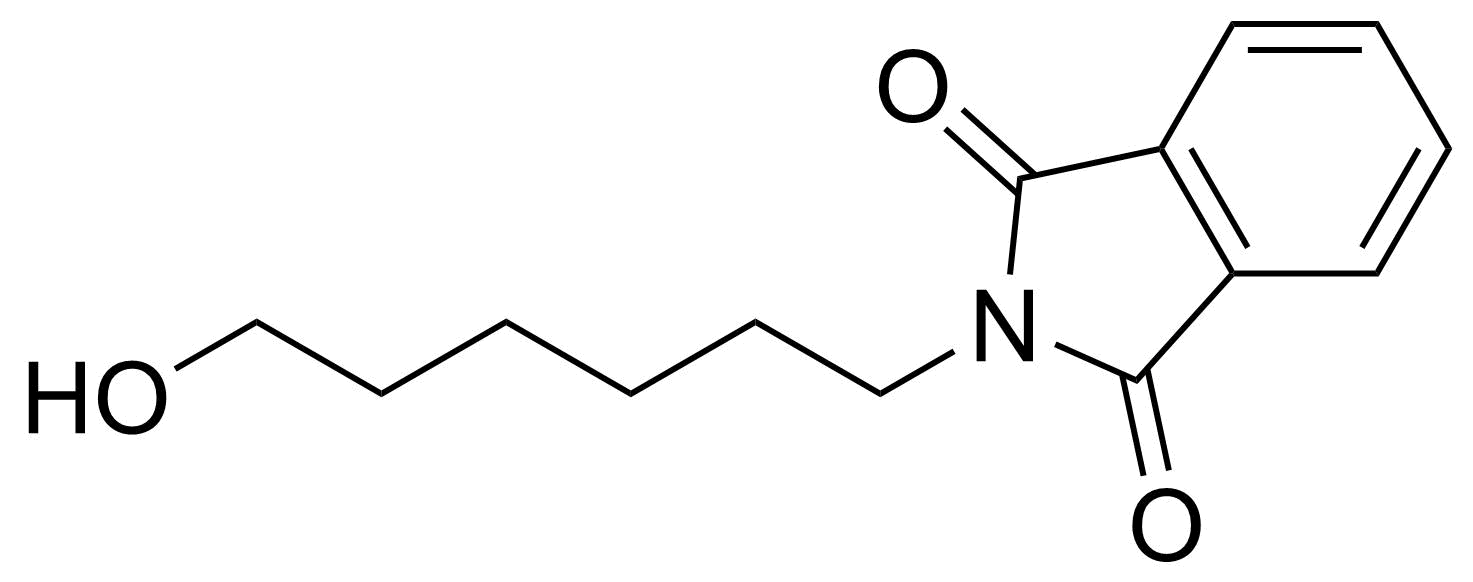
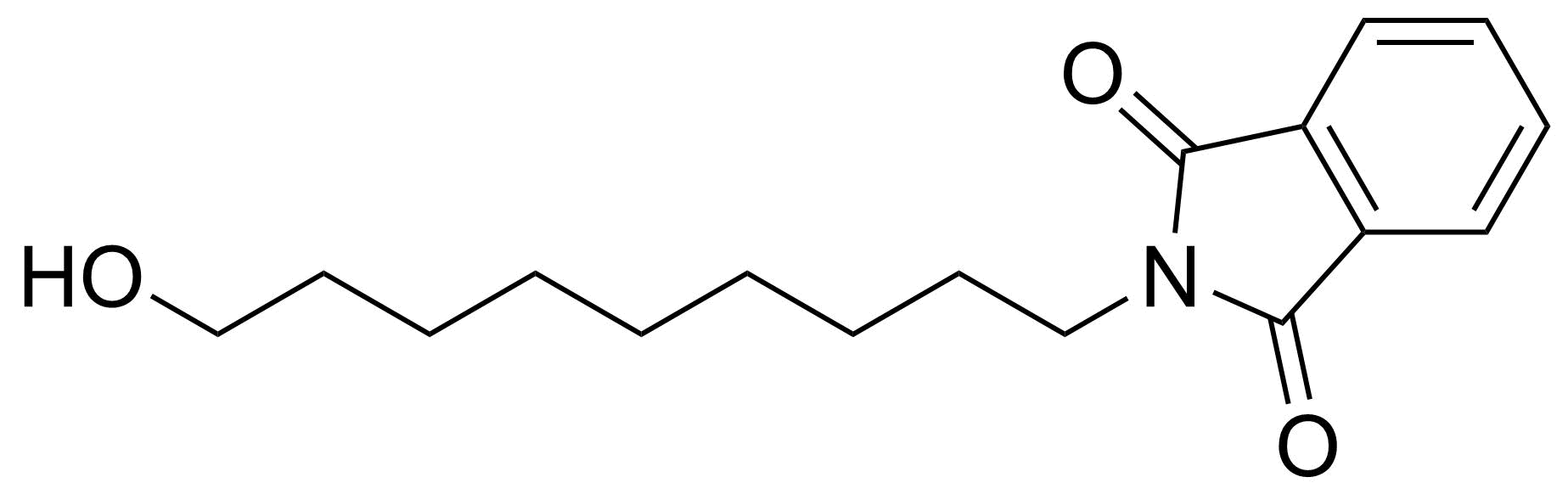
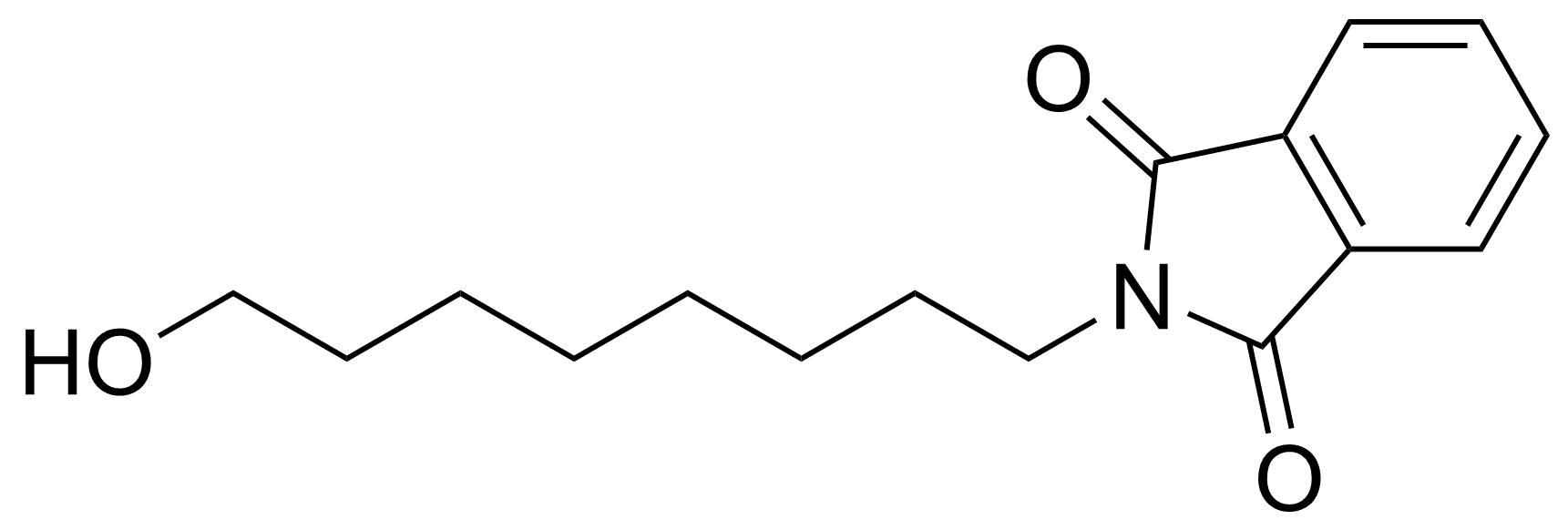
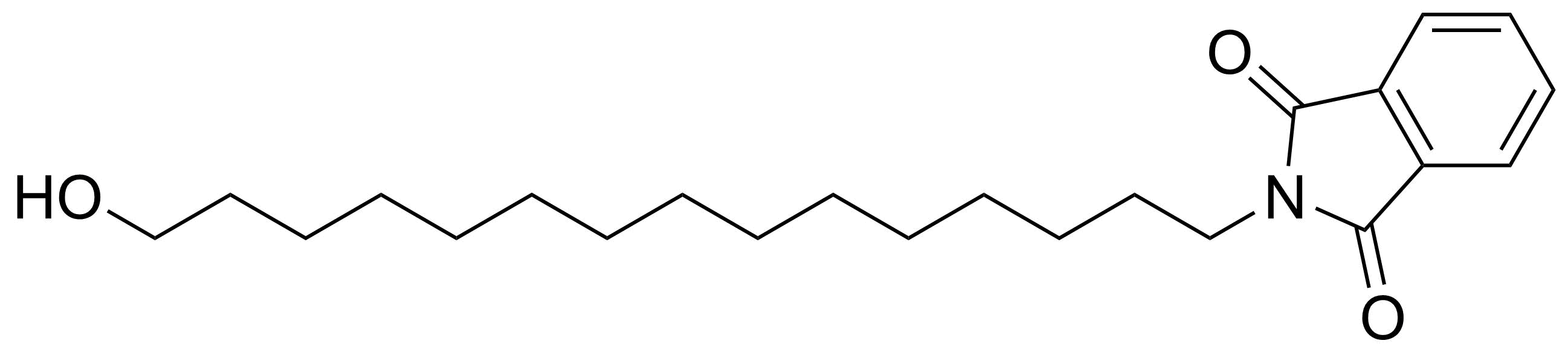
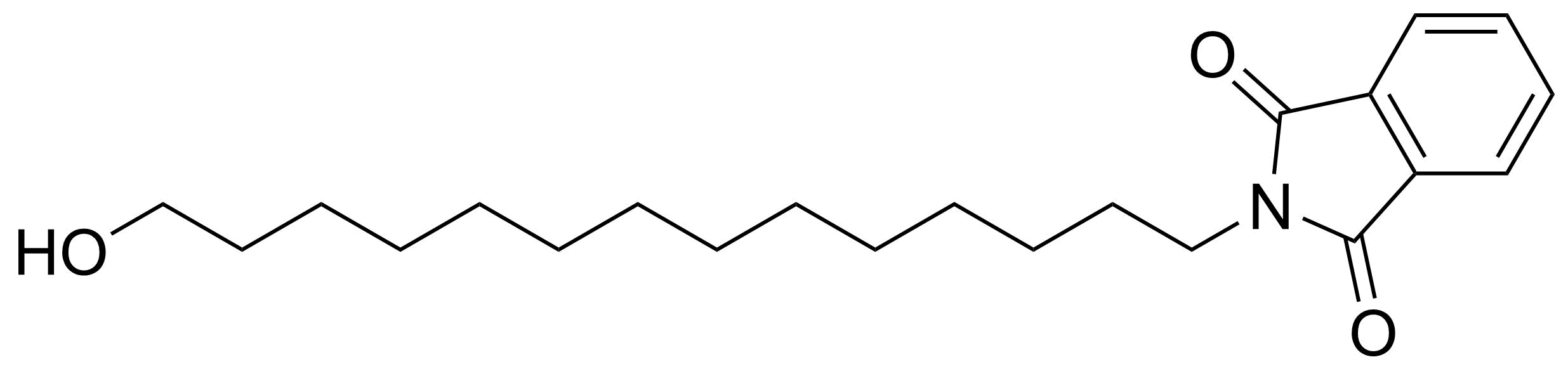
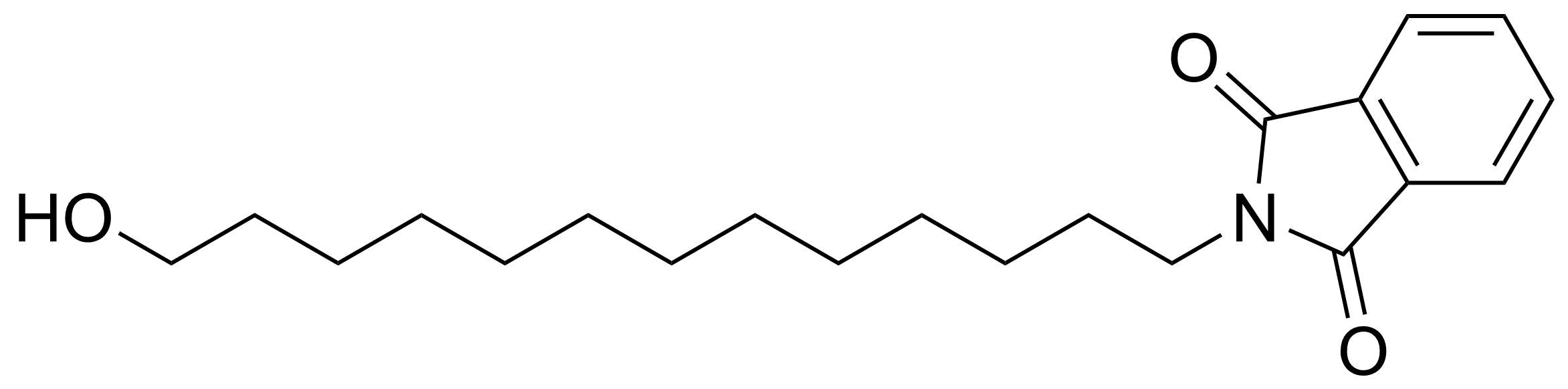
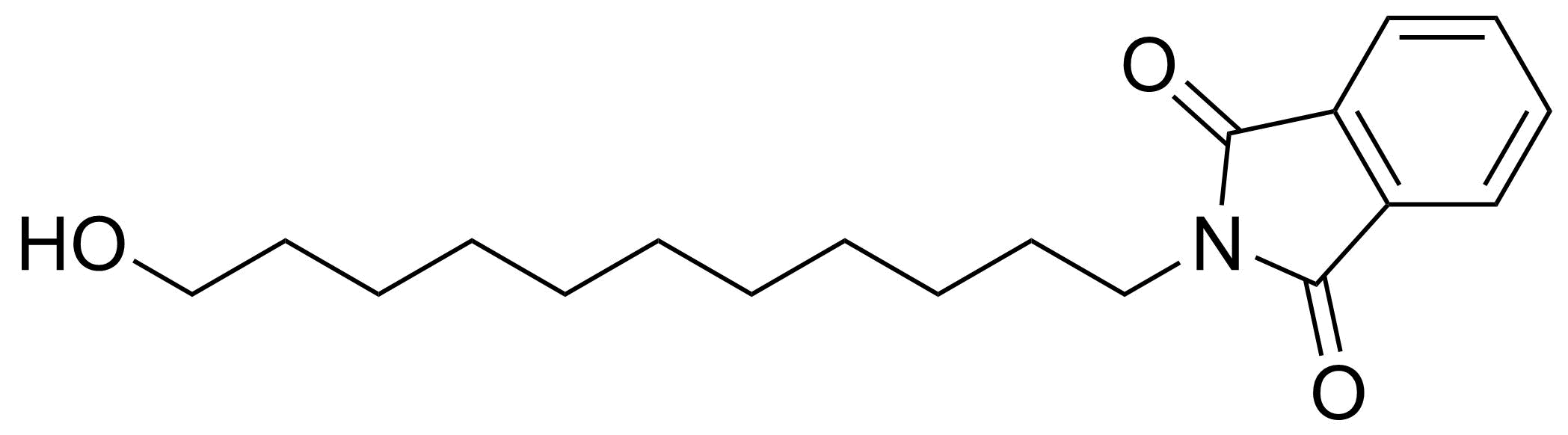
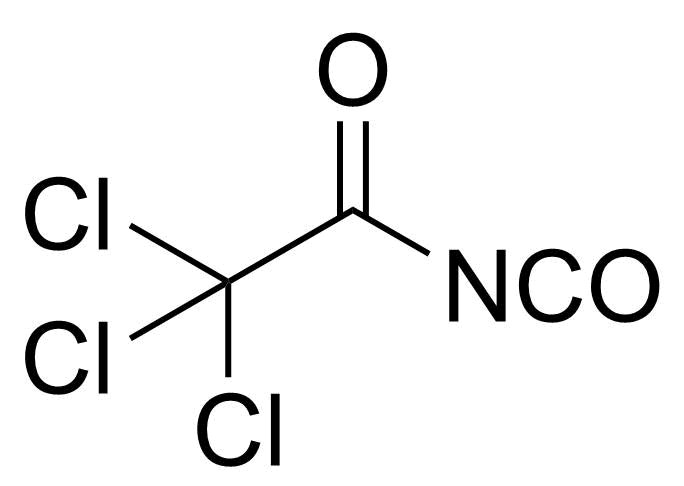
Carboxylic acid derivatives / Anhydrides / Imides / Hydrazides
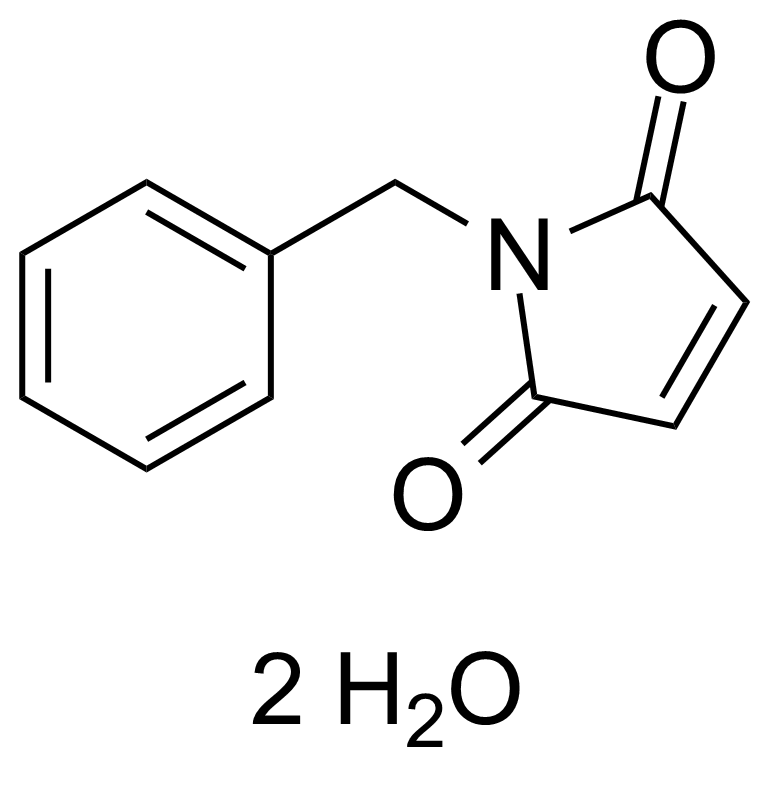
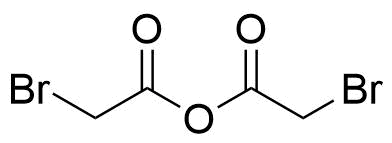
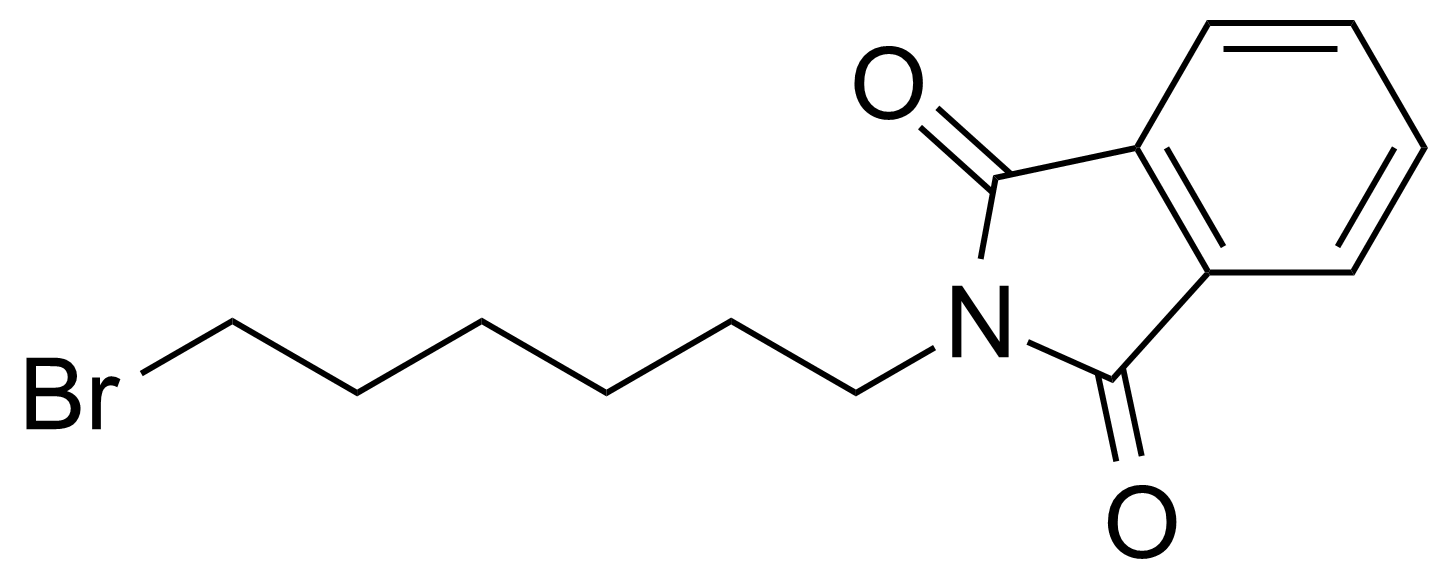
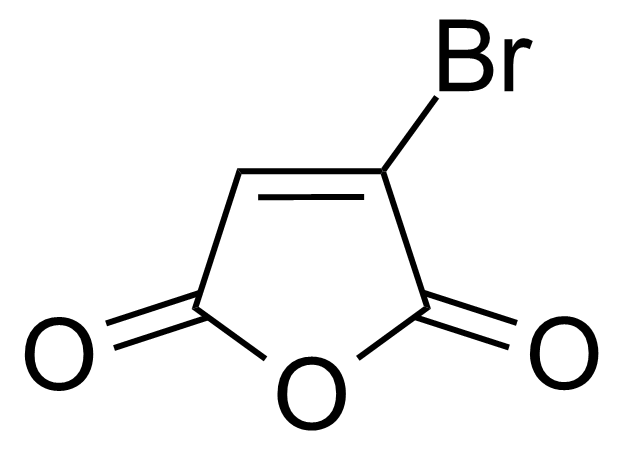
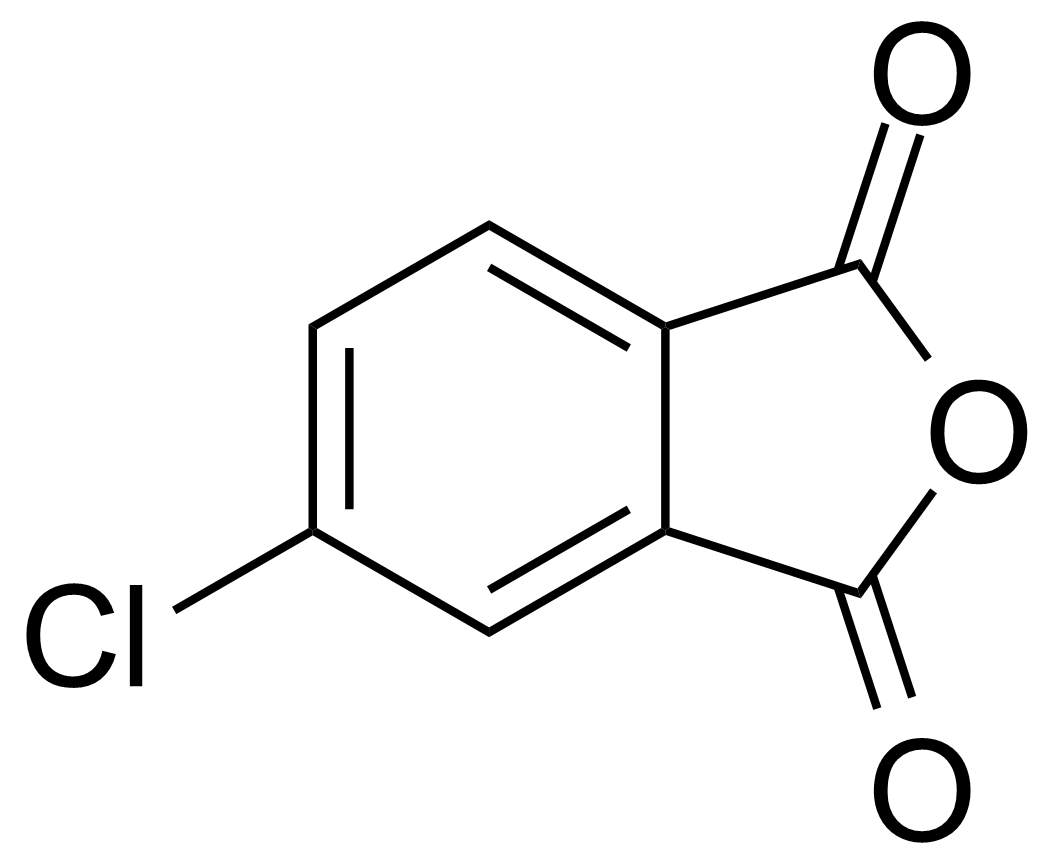
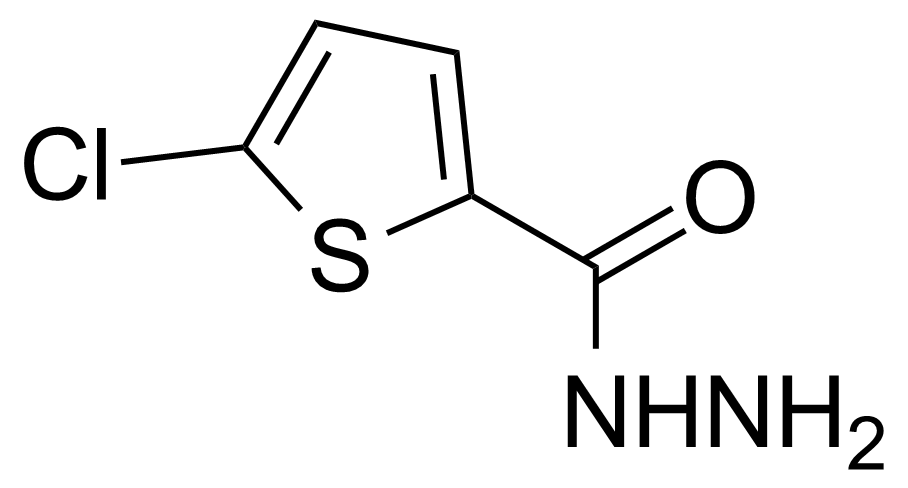
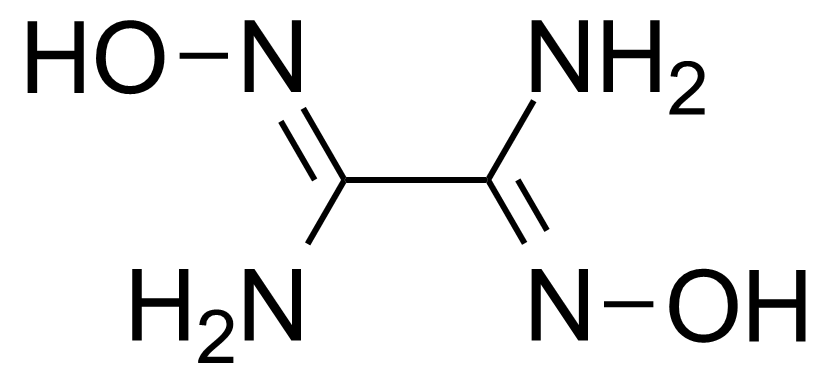
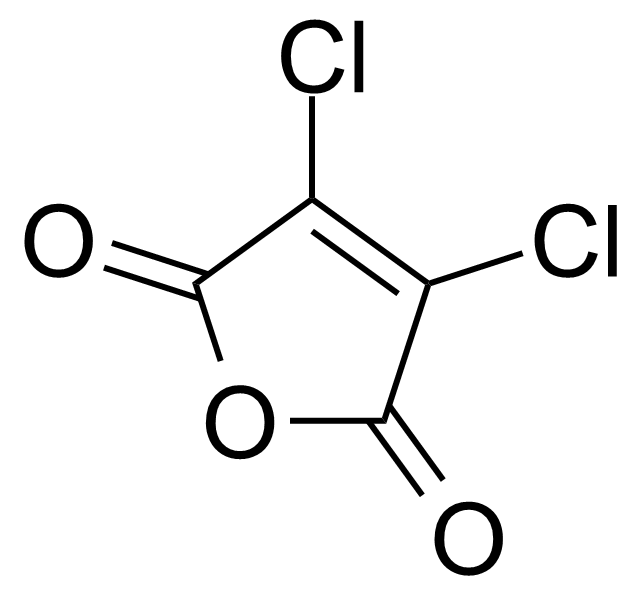
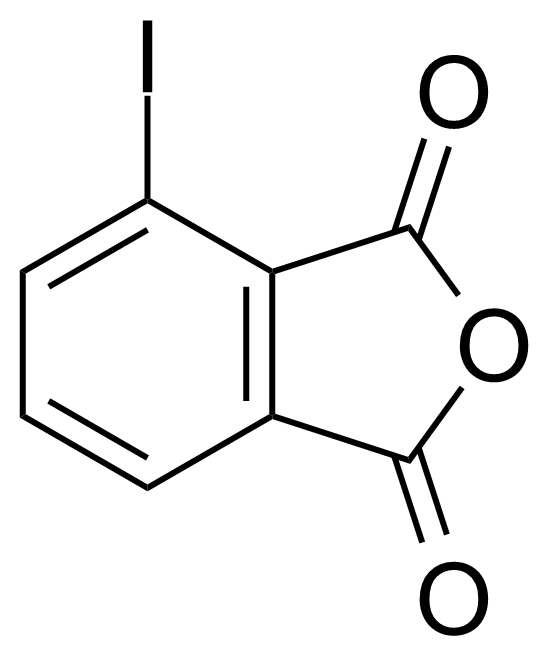
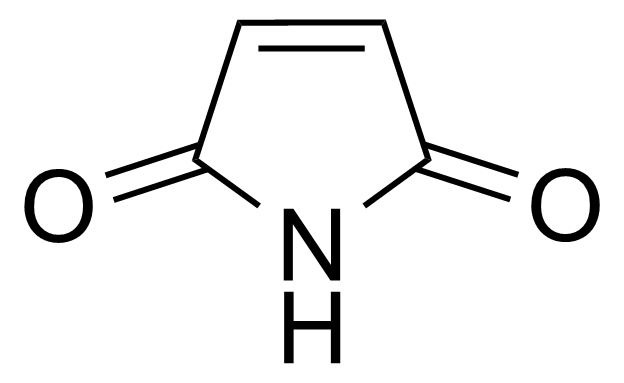
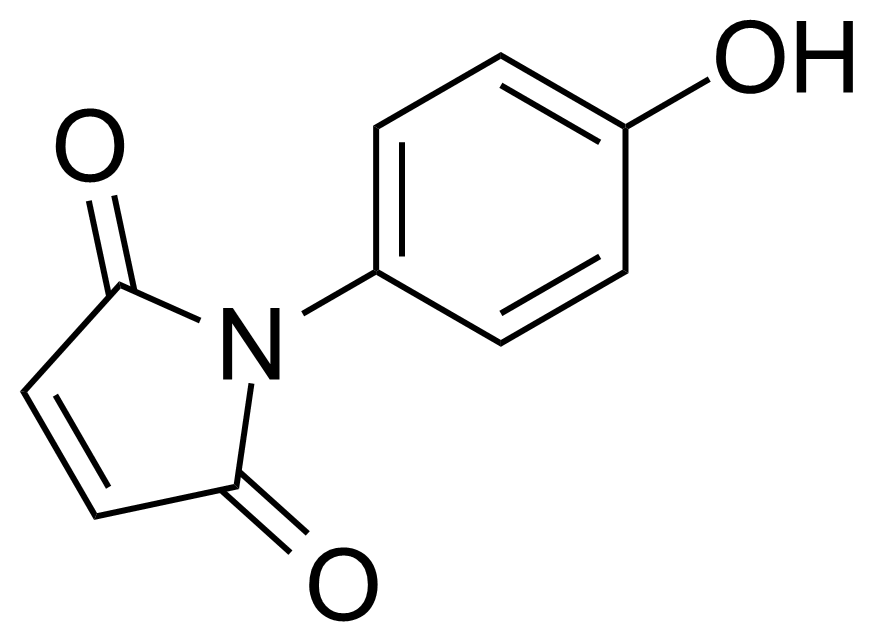
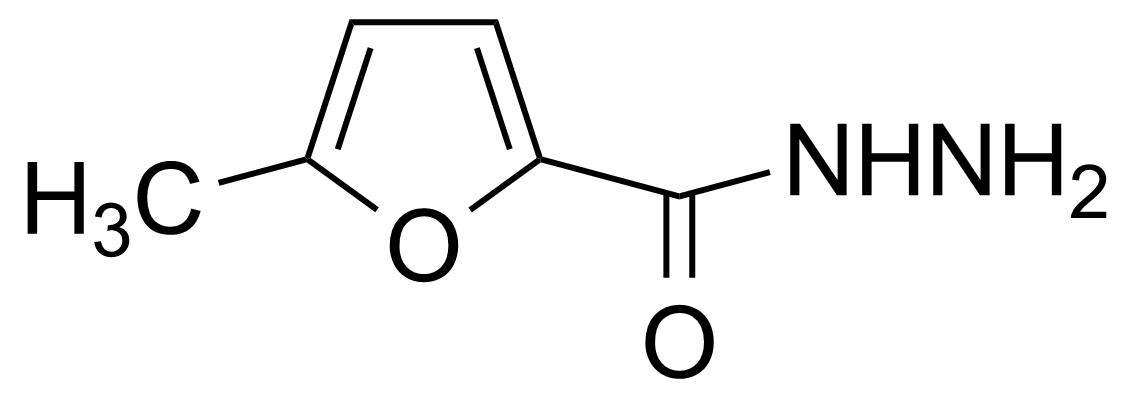
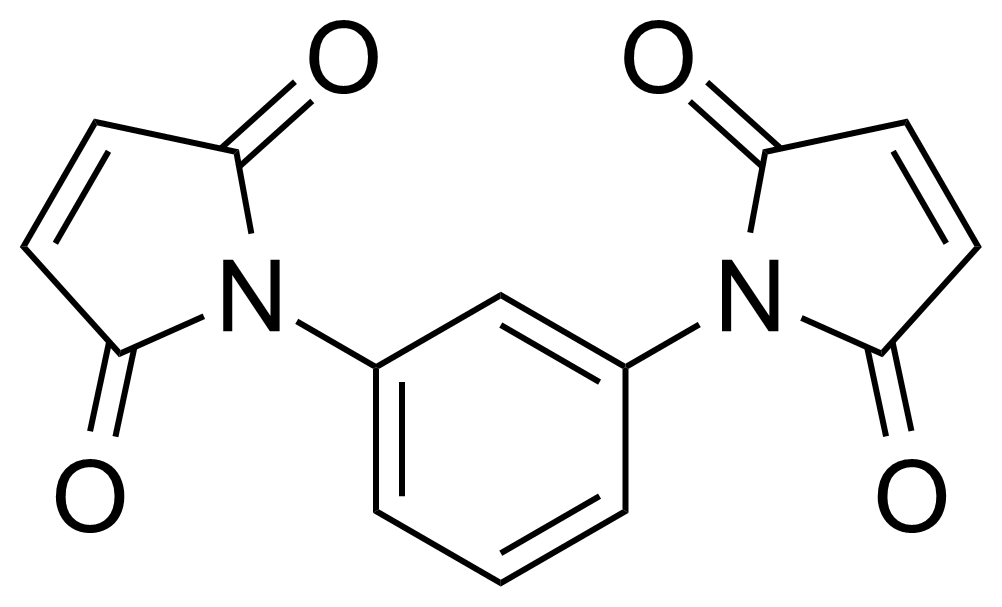
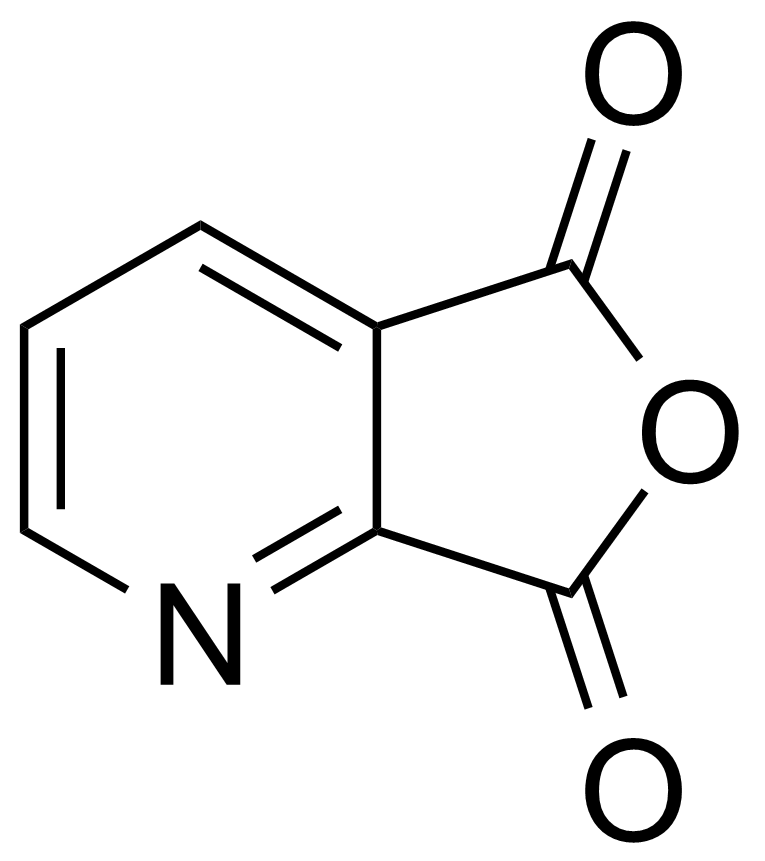
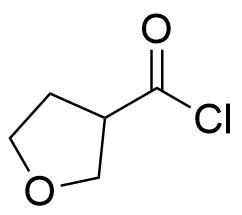
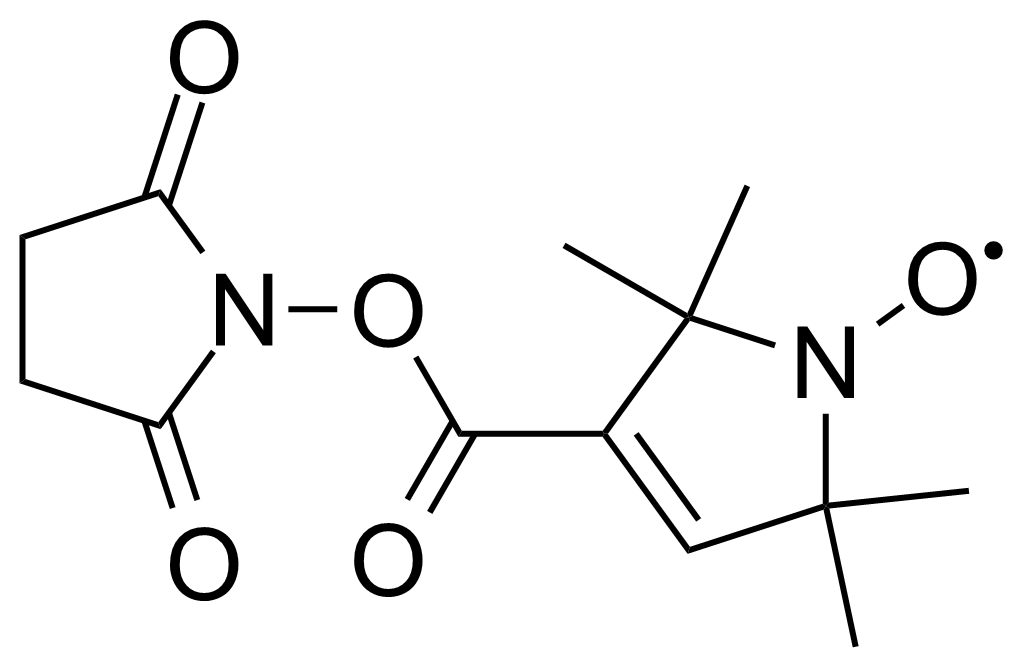
Anhydrides are reactive derivatives of carboxylic acids which have two acyl groups bonded by oxygen atom. Laboratory routes emphasize the dehydration of the corresponding acids. The conditions vary from acid to acid, but phosphorus pentoxide is a common dehydrating agent. Acid chlorides are also effective precursors to anhydrides. Acid anhydrides are a source of reactive acyl groups, and their reactions and uses resemble those of acyl halides however they tend to be less electrophilic. Imides are structurally related to anhydrides although imides are more resistant to hydrolysis. They have two acyl groups are bound to nitrogen atom. Imides are best known in commercial applications as components of high-strength polymers, called polyimides. Acyl hydrazides are derivatives of carboxylic acids, typically prepared by the reaction of esters with hydrazine. Hydrazide analogues have biological activities like antidepressant, anticonvulsant, antiinflammatory, antibacterial, antimalarial, anticancer, and antimicrobial.
![Structure of Benzo[b]thiophene-2-carboxylic hydrazide](https://georganics.sk/wp-content/uploads/2021/05/GEO-00294_Benzobthiophene-2-carboxylic_hydrazide.png)