In stock
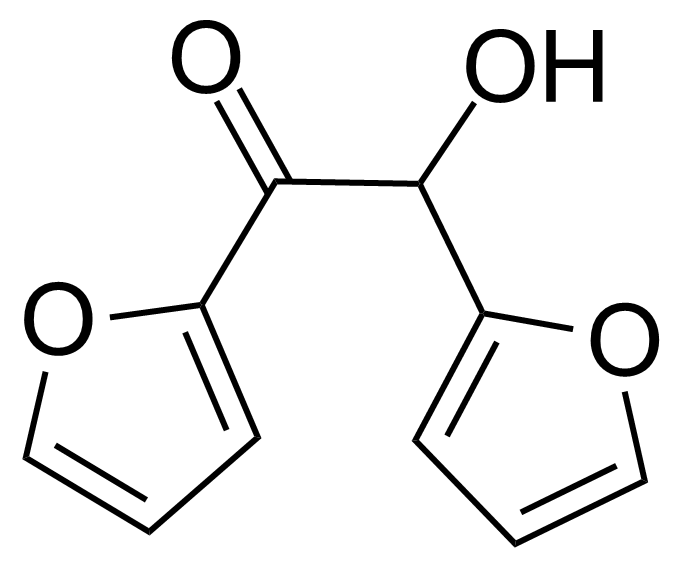
Furoin
CAS#[552-86-3]
G-codeGEO-01452
EC number209-024-8
Molecular formulaC10H8O4
Molecular weight192,17
Synonyms
2,2'-Furoin ; Furoin (mixture of isomers) ; 1,2-di-(2-Furanyl)ethan-1-one ; alpha-Furoin ; (+/-)-2-Furoin ;
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
Documents
Product categorization
Second level
Third level
Description
Furoin is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Furoin in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Furoin can be used in synthesis of pyrazine and quanoxiline derivatives by …
Show full descriptionGeneral description and preparation:
Furoin or 1,2-di(furan-2-yl)-2-hydroxyethanone [552-86-3] is a white crystalline solid with the melting point of 136-137 °C.[1] It can be produced from furfural via benzoin condensation in presence of cyanide ion[2] or N-heterocyclic carbene catalyst as various thiazolium and imidazolium ions.[3] They can be further supported as a recyclable precatalysts, which upon treatment with a base, catalyze furfural self-condensation coupling reaction into furoin.[4] Furoin synthesis from furfural is also catalyzed by vitamin B1 (thiamine). In 1957, Ronald Breslow proposed the mechanism of reaction that involves a relatively stable carbene form of thiamine, which was the first evidence for the existence of persistent carbenes.[5]Application of Furoin:
Furoin can be used in synthesis of pyrazine and quanoxiline derivatives by condensation with diamines.[6] Different tetrasubstituted pyroles can be prepared from furoin as α-hydroxy ketone via a base-promoted three-component reaction with malononitrile and alcohols[7] or 1,3-dicarbonyls and ammonium acetate.[8]Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________ [1] L. Myles, N. Gathergood, S. J. Connon Chem. Commun. 2013, 49, 5316. doi:10.1039/C3CC41588K [2] W. W. Hartman, J. B. Dickey J. Am. Chem. Soc. 1933, 55 (3), 1228. doi:10.1021/ja01330a063 [3] H. Sugimoto, K. Hirai Tetrahedron Lett. 1985, 26 (7), 883. doi:10.1016/S0040-4039(00)61955-X [4] L. Wang, E. Y. X. Chen Green. Chem. 2015,17, 5149. doi:10.1039/C5GC01648G [5] R. Breslow J. Am. Chem. Soc. 1958, 80 (14), 3719. doi:10.1021/ja01547a064 [6] W. Song, P. Liu, H. You, X. Chen, H. Chen, L. Ma, L. Hu Synth. Commun. 2012, 42 (2), 236. doi:10.1080/00397911.2010.523489 [7] H. Liu, C. Qi, L. Wang, Y. Guo, D. Li, H. Jiang J. Org. Chem. 2021, 86, 9610. doi:0.1021/acs.joc.1c00882 [8] S. I. Bjat, D. R. Trivedi Tetrahderon Lett. 2013, 54 (41), 5577. doi:10.1016/j.tetlet.2013.07.153
Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| New | 1-Acenaphthenol | 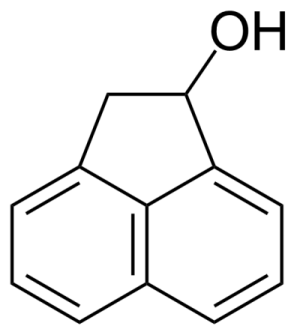 | [6306-07-6] | GEO-00001 |
| New | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 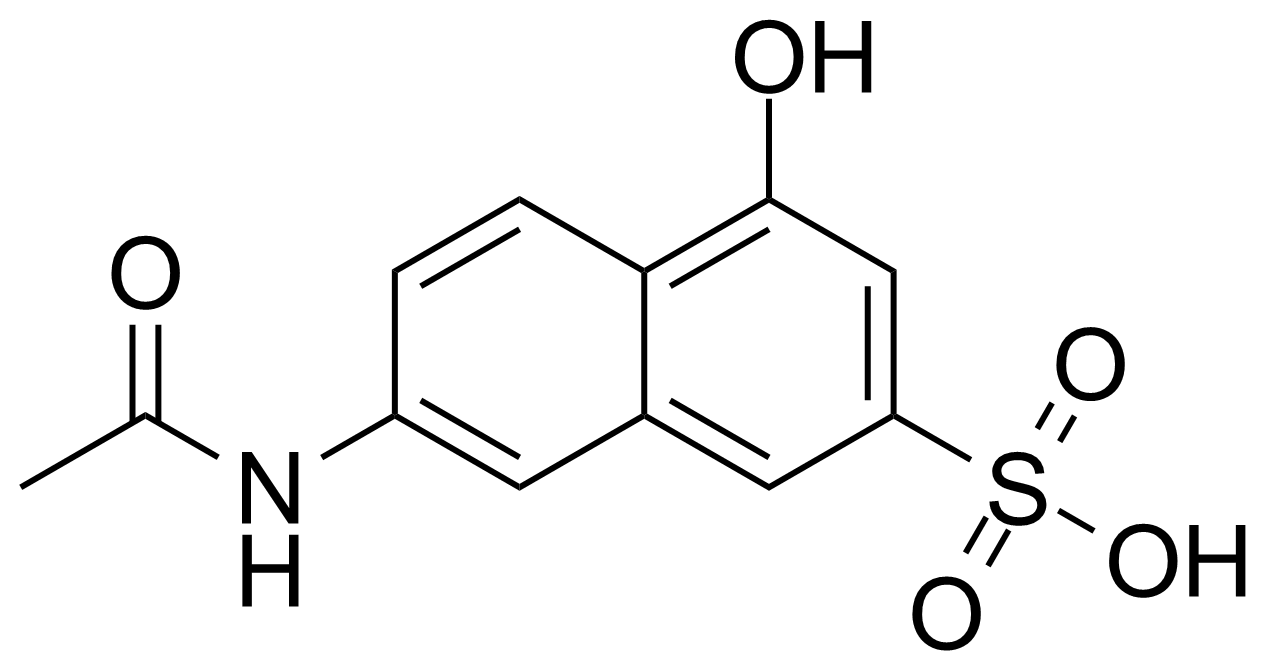 | [6334-97-0] | GEO-04013 |
| New | 1-Adamantanemethanol | 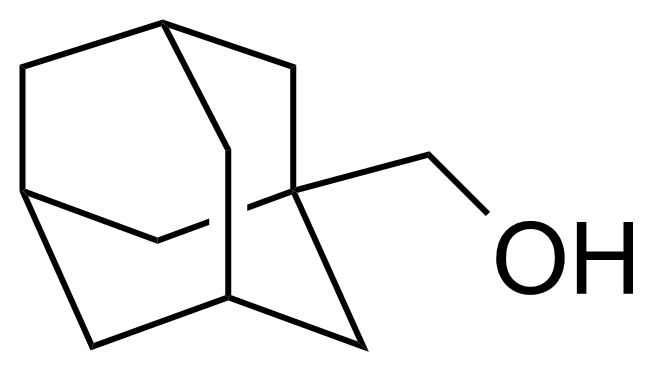 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 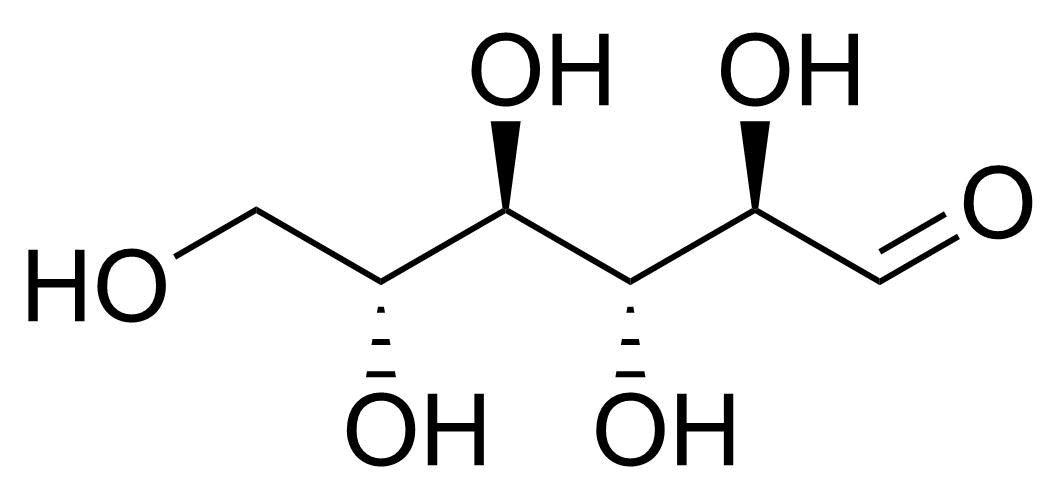 | [7283-09-2] | GEO-04660 | |
| New | D-Allose | 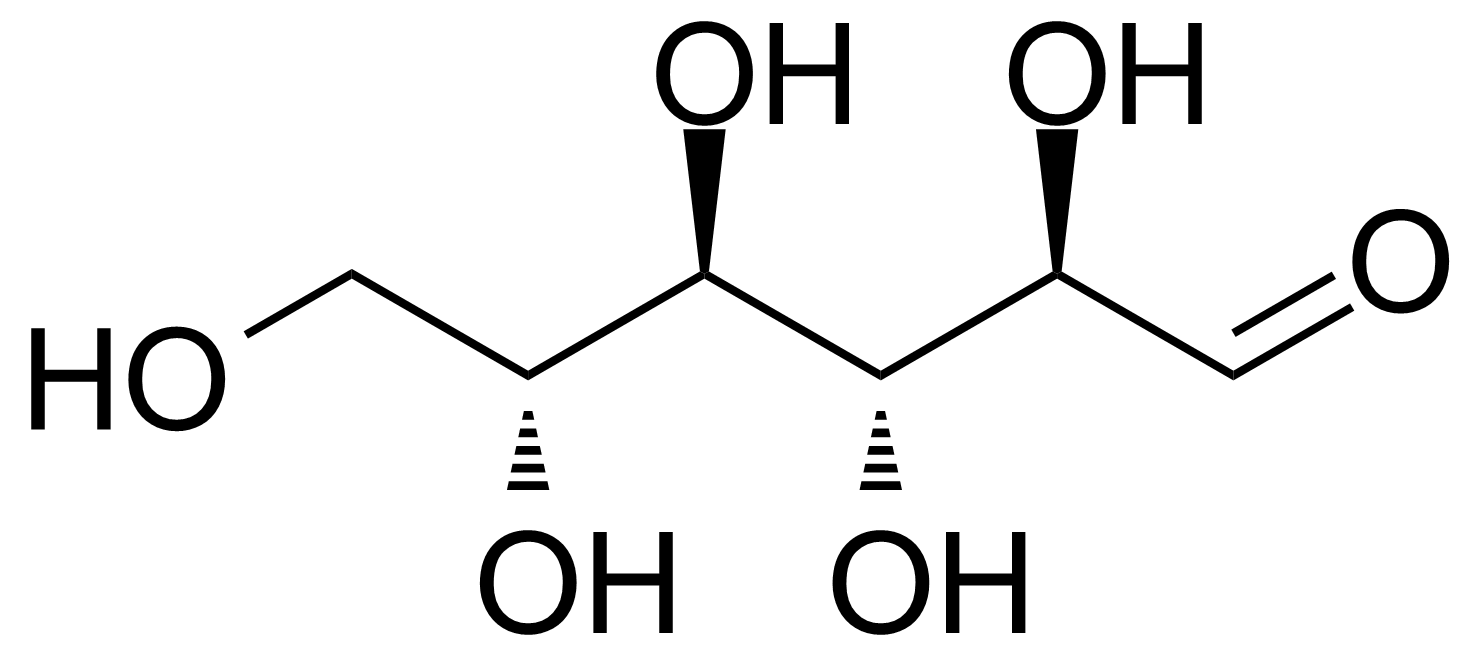 | [2595-97-3] | GEO-00057 |
| L-Allose | 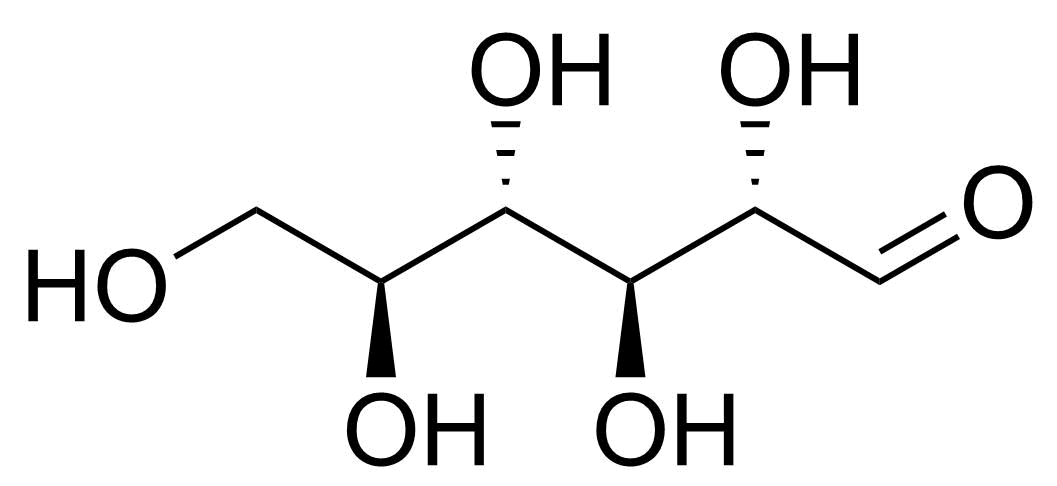 | [39392-62-6] | GEO-04661 | |
| New | 2-(Allyloxy)phenol | 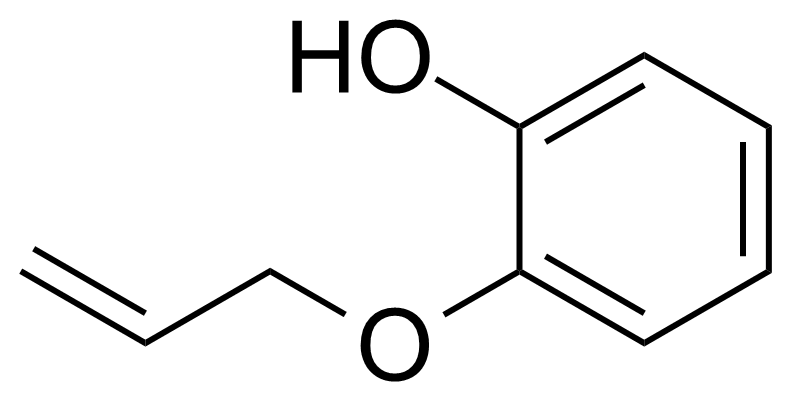 | [1126-20-1] | GEO-04471 |
| D-Altrose | 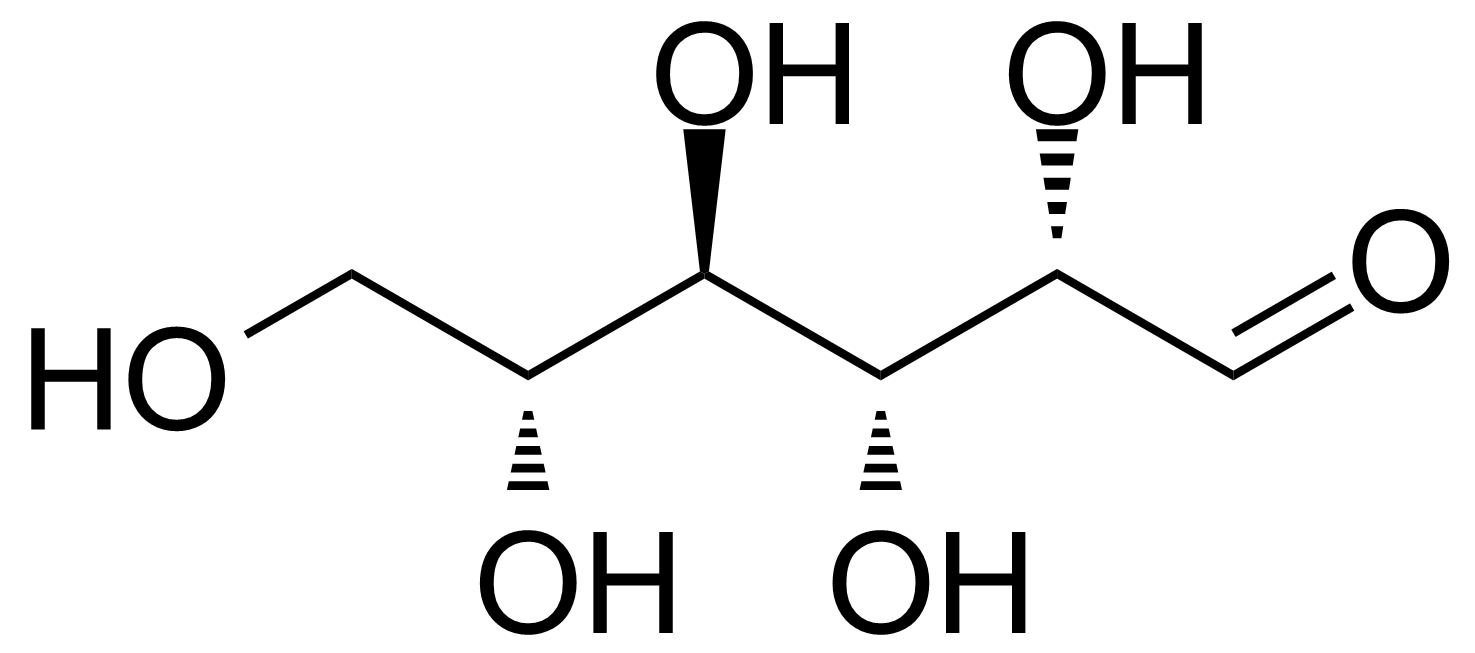 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 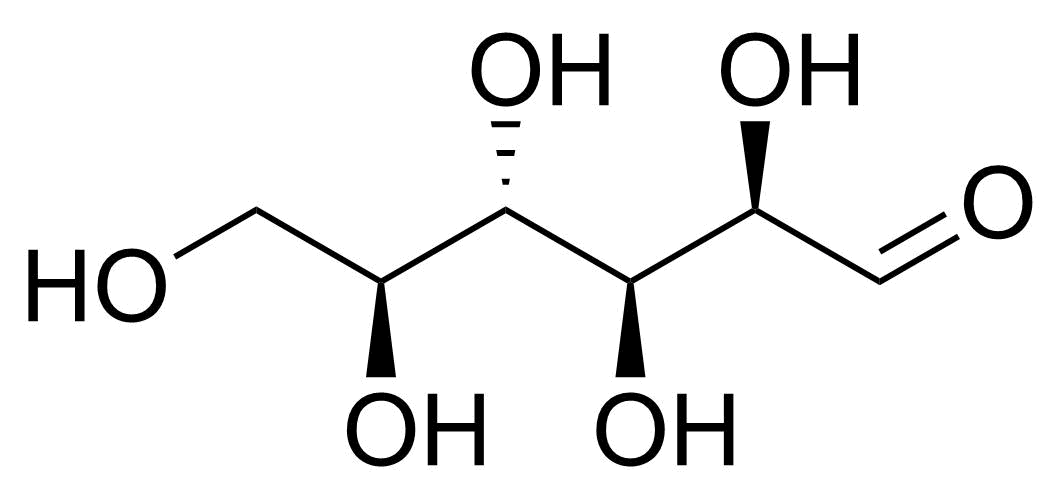 | [1949-88-8] | GEO-04662 | |
| New | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 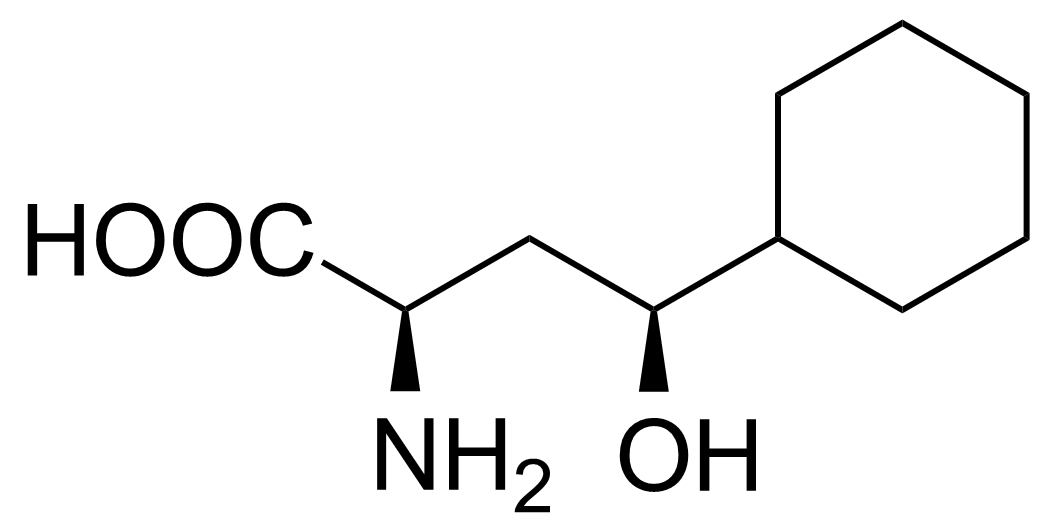 | [] | GEO-02717 |