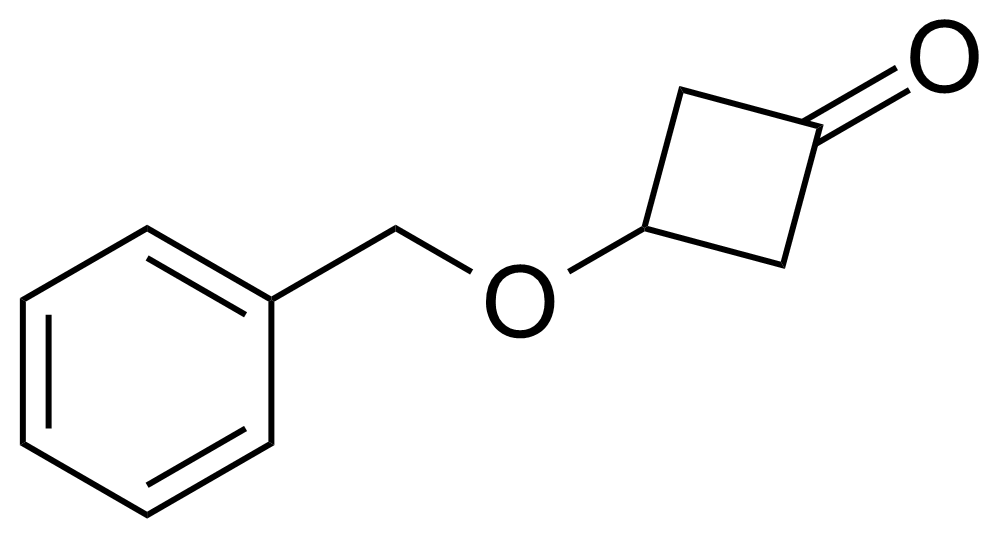
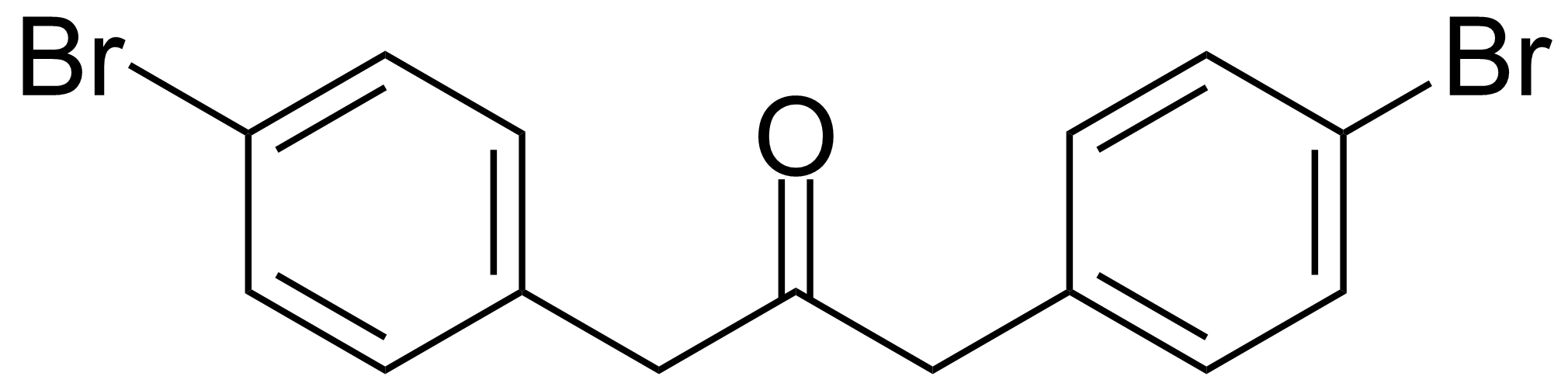
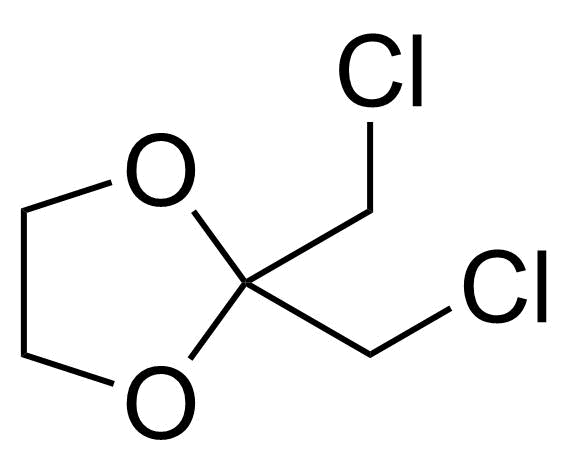
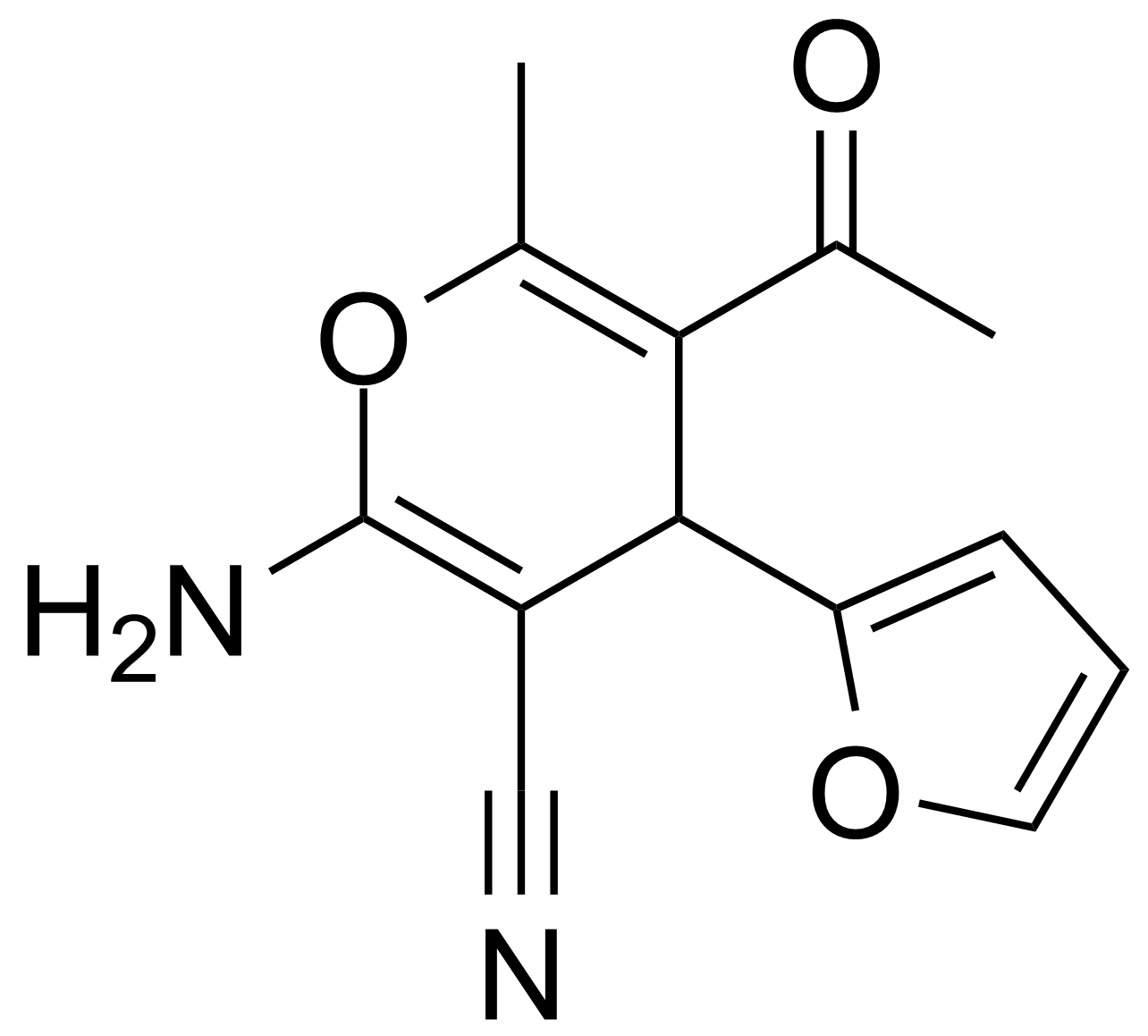
Ketones / Ketals / Aminals
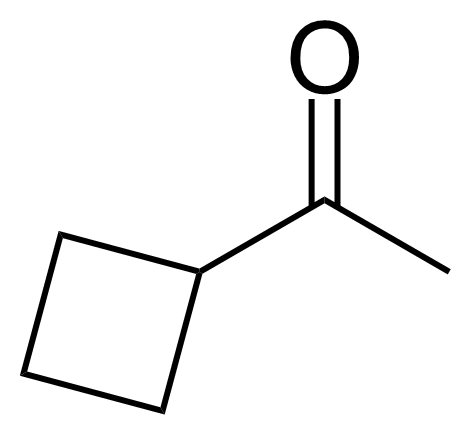
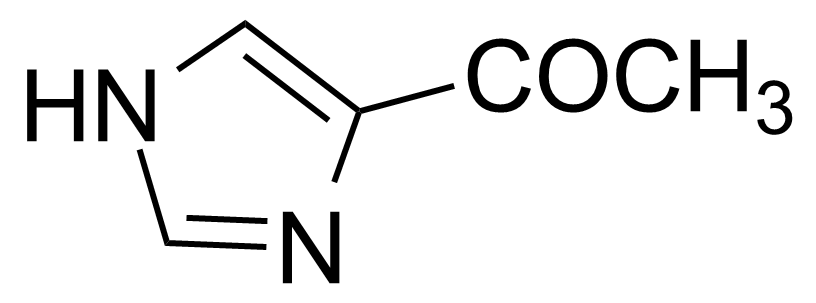
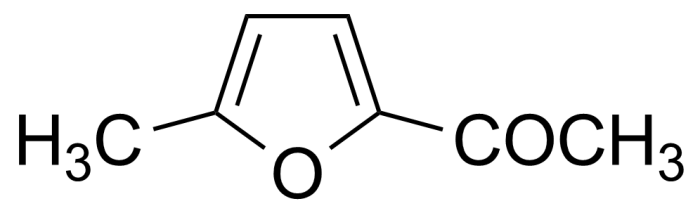
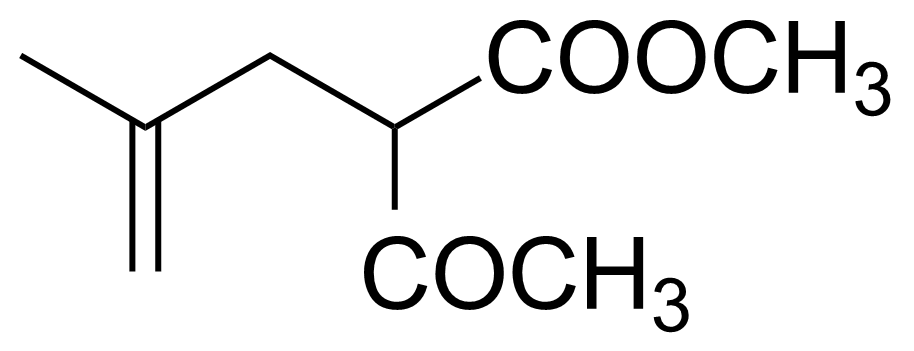
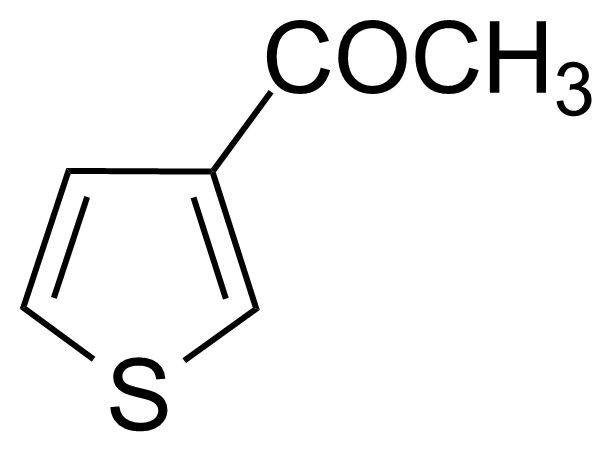
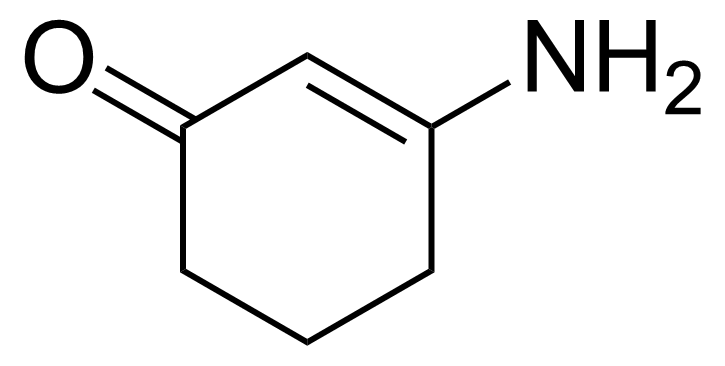
Ketones are important group of organic compounds that contain carbonyl function group substituted by alkyl chain from both sides and therefore they can be symmetric, asymmetric and also cyclic. Ketones can be prepared by many industrial and laboratory methods. In industry, the most important method probably involves oxidation of hydrocarbons. For a small scale organic synthetic application, ketones are often prepared by oxidation of secondary alcohols. Typical strong oxidants include potassium permanganate, Cr (VI) compounds, milder ones as Dess–Martin periodinane or the Swern methods. Many other methods of preparation exist (hydrolysis of halides, hydration of alkynes, Weinreb–Nahm ketone synthesis, Friedel–Crafts acylation, ozonolysis, Nef reaction etc.). They engage in many organic reactions. The most important reactions follow from the susceptibility of the carbonyl carbon toward nucleophilic addition and the tendency for the enolates to add to electrophiles. Ketones are pervasive in nature, many sugars are ketones, known collectively as ketoses. The best-known ketose is fructose. Ketals are acetals of ketones while aminals or amino acetals are organic compound that has two amine groups attached to the same carbon atom. Aminals are encountered in, for instance, the Fischer indole synthesis.
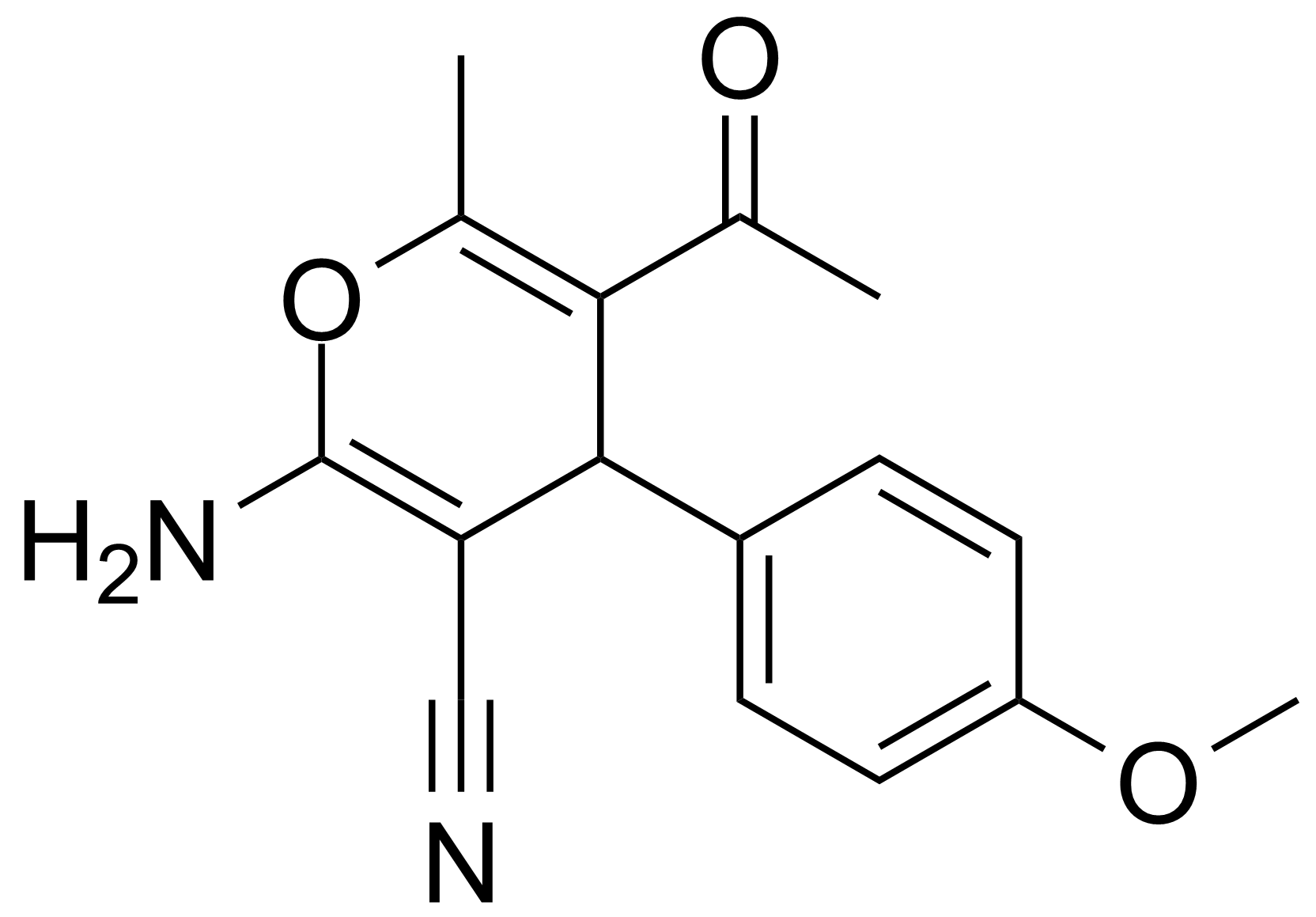
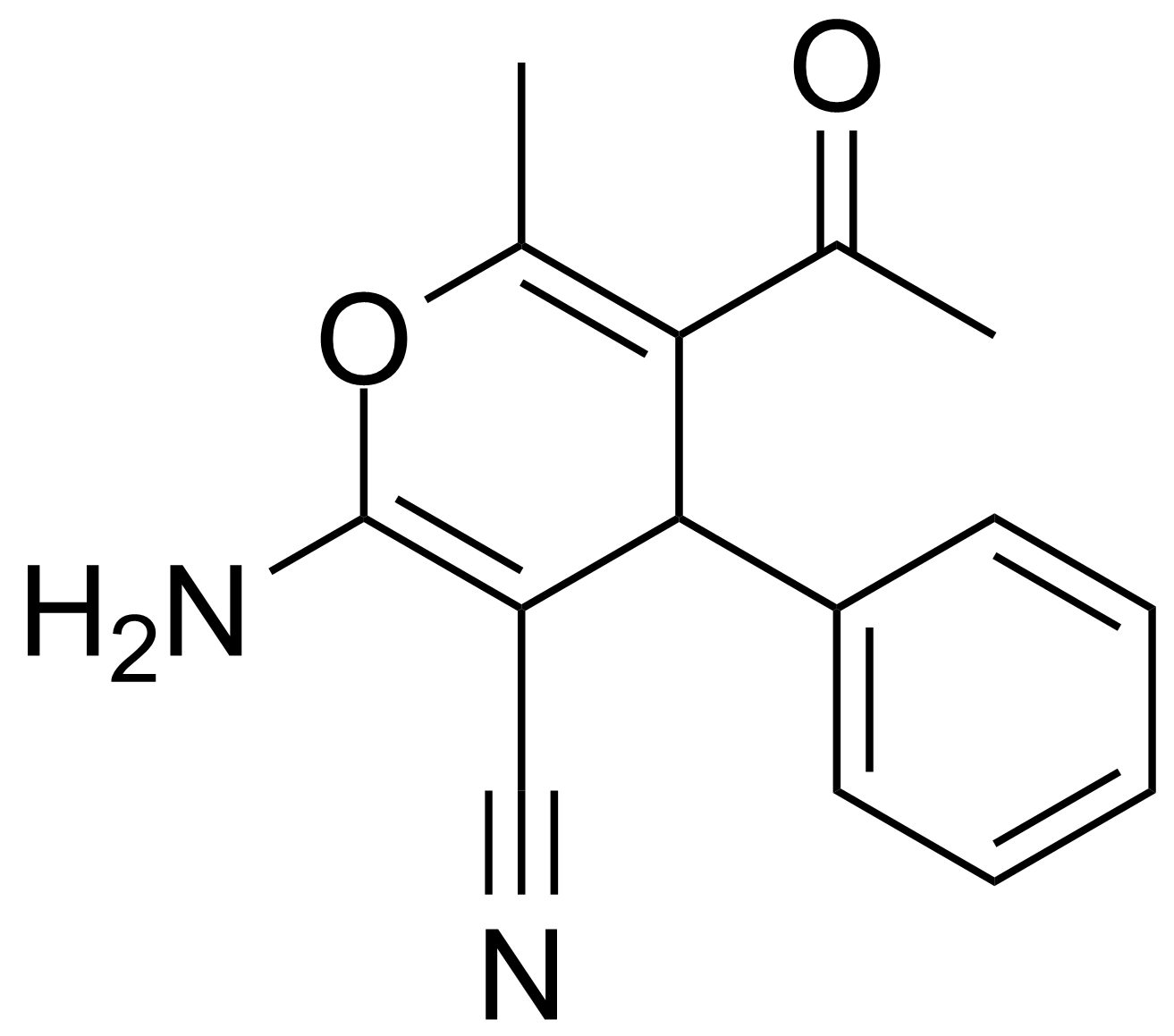
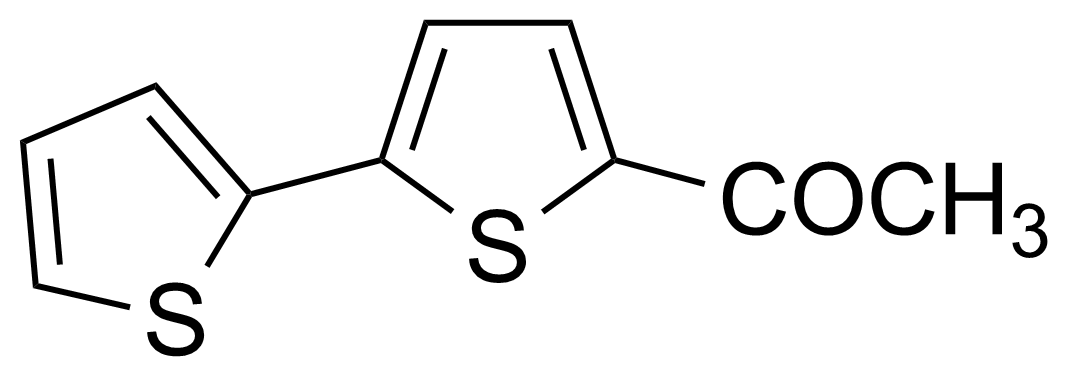
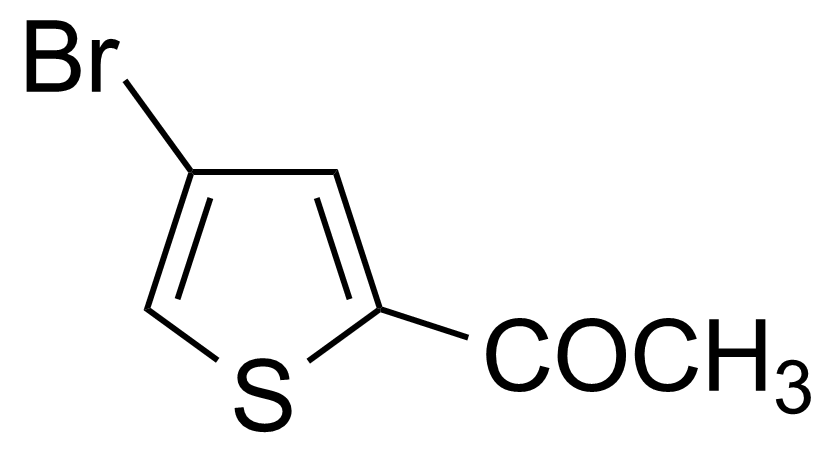
![Structure of 5-Acetyl-2,3-dihydrobenzo[b]furan](https://georganics.sk/wp-content/uploads/2021/05/GEO-00030_5-Acetyl-23-dihydrobenzobfuran.png)
![Structure of (3-Amino-4,6-dimethyl-furo[2,3-b]pyridin-2-yl)-p-tolyl-methanone](https://georganics.sk/wp-content/uploads/2021/05/GEO-02695_3-Amino-46-dimethyl-furo23-bpyridin-2-yl-p-tolyl-methanone.png)