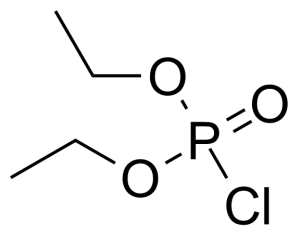
Diethyl chlorophosphate
Diethylphosphoryl chloride ; Diethyl chlorophosphonate ; O,O-Diethyl phosphorochloridate ; Phosphoric acid diethyl ester chloride ; Phosphorochloridic acid O,O-diethyl ester ; Diethyl phosphorochloridate
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H300 – Fatal if swallowed.
H310 – Fatal in contact with skin.
H331 – Toxic if inhaled.
P262 – Do not get in eyes, on skin, or on clothing.
P264 – Wash … thoroughly after handling.
P280 – Wear protective gloves/protective clothing/eye protection/face protection.
P301+P310 – IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.
P302+P352+P310 – IF ON SKIN: Wash with plenty of water. Immediately call a POISON CENTER/ doctor.
P304+P340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P311 – Call a POISON CENTER or doctor/physician.
Product categorization
Description
Diethyl chlorophosphate is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Diethyl chlorophosphate in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
By using Diethyl chlorophosphate, some ketones can be converted to enol phosphates which can be reduced to alkenes/alkanes or coupled with…
Show full descriptionGeneral description of Diethyl chlorophosphate:
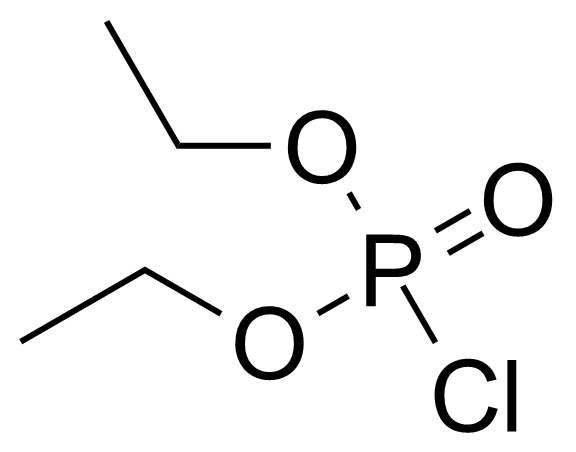
 Diethyl chlorophosphate [814-49-3] or diethyl phosphorochloridate is a colorless to faith yellow clear liquid with the fruity odor and the boiling point of 60 °C/2 mmHg.[1] It acts as an cholinesterase inhibitor. It has high oral (LD50 = 11 mg/kg, rat) and very high dermal toxicity (LD50 = 8 μL/kg, rabbit), it is also toxic by inhalation.[2] This compound is typically prepared by the chlorination of diethylphosphite with carbon tetrachloride, which is called Atherton–Todd reaction.[3] Another option of preparation is reaction of phosphoryl chloride with ethanol in the presence of triethylamine.[4]
Diethyl chlorophosphate [814-49-3] or diethyl phosphorochloridate is a colorless to faith yellow clear liquid with the fruity odor and the boiling point of 60 °C/2 mmHg.[1] It acts as an cholinesterase inhibitor. It has high oral (LD50 = 11 mg/kg, rat) and very high dermal toxicity (LD50 = 8 μL/kg, rabbit), it is also toxic by inhalation.[2] This compound is typically prepared by the chlorination of diethylphosphite with carbon tetrachloride, which is called Atherton–Todd reaction.[3] Another option of preparation is reaction of phosphoryl chloride with ethanol in the presence of triethylamine.[4]Application of Diethyl chlorophosphate:
By using this substance, some ketones can be converted to enol phosphates which can be reduced to alkenes/alkanes or coupled with organometallic reagents to form substituted alkenes. Following enol phosphates can be then converted into β-keto phosphonates, useful for Horner-Emmons homologation.[5] It is also used in the synthesis of organophosphorus nerve agent mimics.[6] Phosphoroamidate linkages are found in a large array of biologically active natural products for example Microcin C7, Dinogunellin, Phosphoarginine, Phosphocreatine, Phosphoramidon, Phosmidosine and Agrocin.[7]Product categorization (Chemical groups):
Main category: ______________________________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 2-Aminoethyl dihydrogen phosphate | 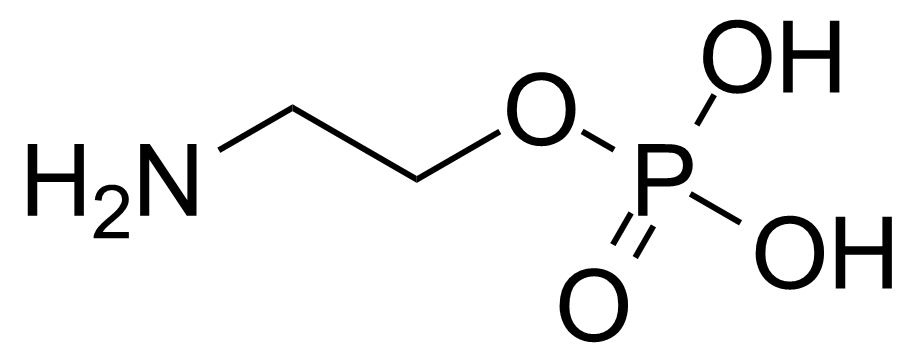 | [1071-23-4] | GEO-00129 | |
| 2-Aminoethyl dihydrogen phosphate magnesium salt (2:1) | 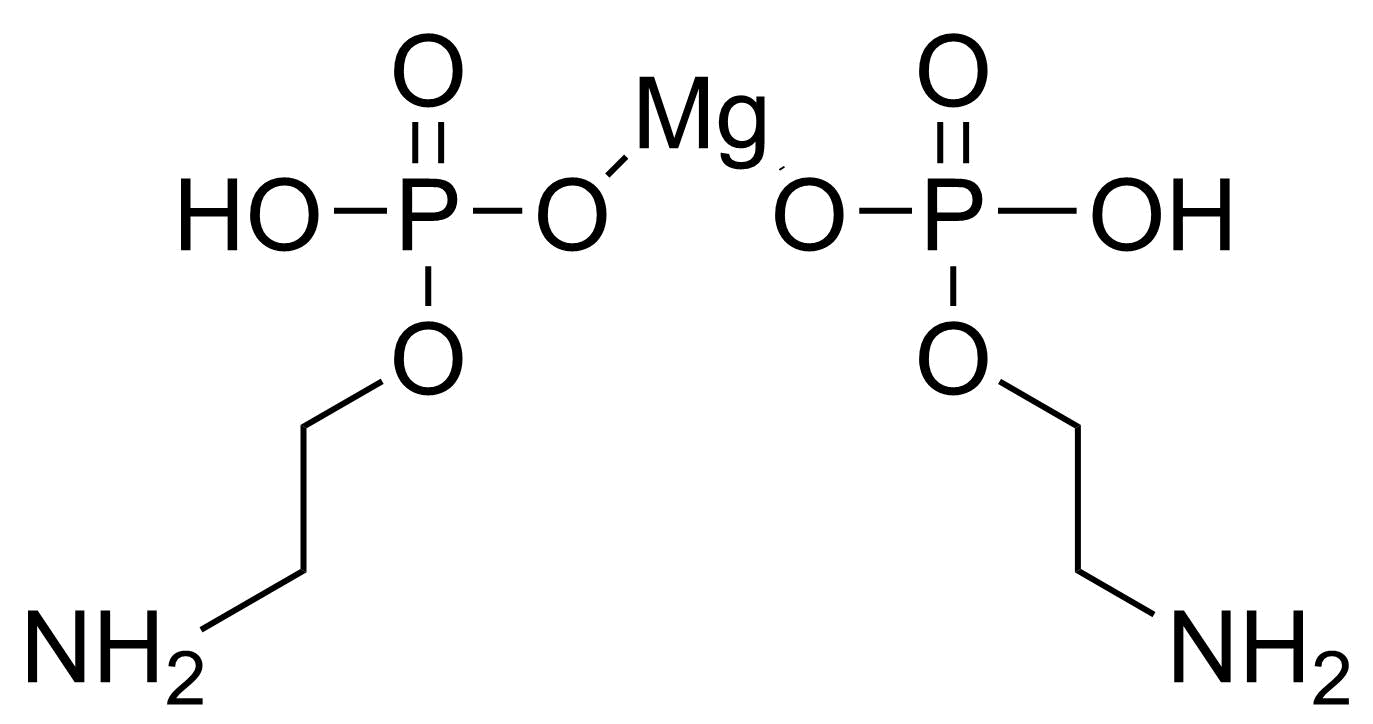 | N/A | GEO-04286 | |
| (Aminomethyl)phosphonic acid | 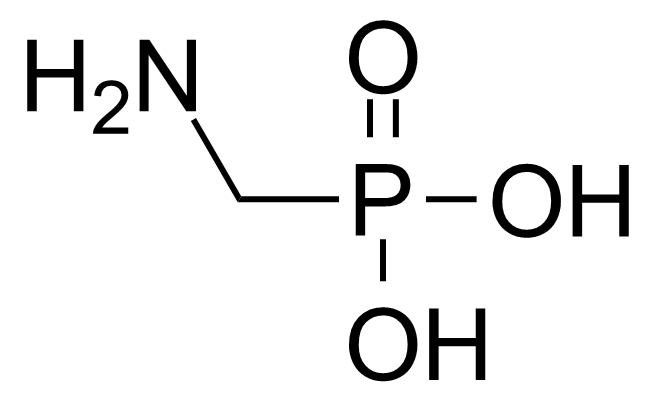 | [1066-51-9] | GEO-00169 | |
| (3-Aminopropyl)phosphonic acid | 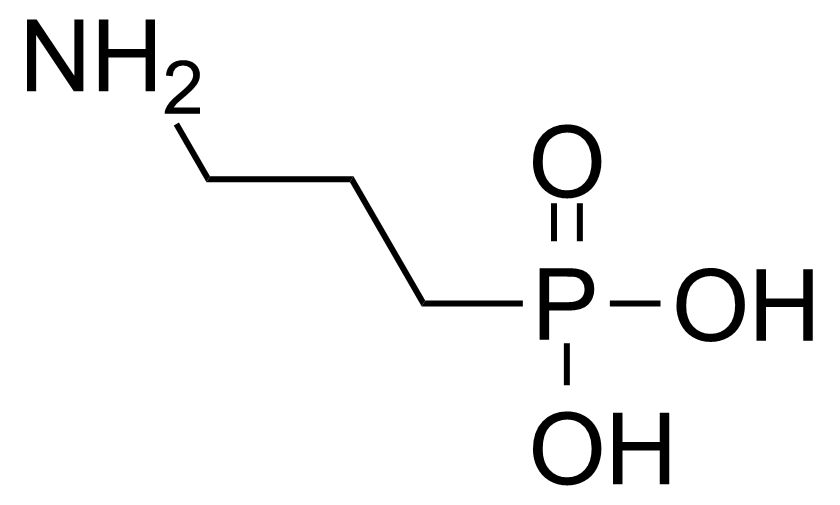 | [13138-33-5] | GEO-00218 | |
| Benzo[d]isoxazol-3-yl diphenyl phosphate | ![Structure of Benzo[d]isoxazol-3-yl diphenyl phosphate](https://georganics.sk/wp-content/uploads/2021/05/GEO-00266_Benzodisoxazol-3-yl_diphenyl_phosphate.png) | [94820-31-2] | GEO-00266 | |
| Benzyl dimethylphosphonoacetate | 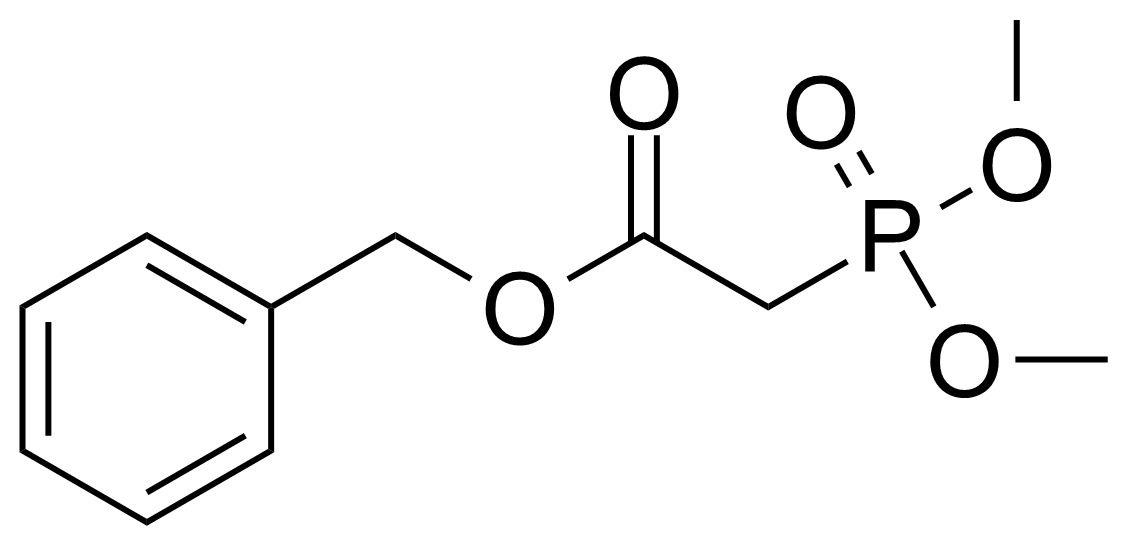 | [57443-18-2] | GEO-02882 | |
| Benzyl(triphenylphosphoranylidene)acetate | 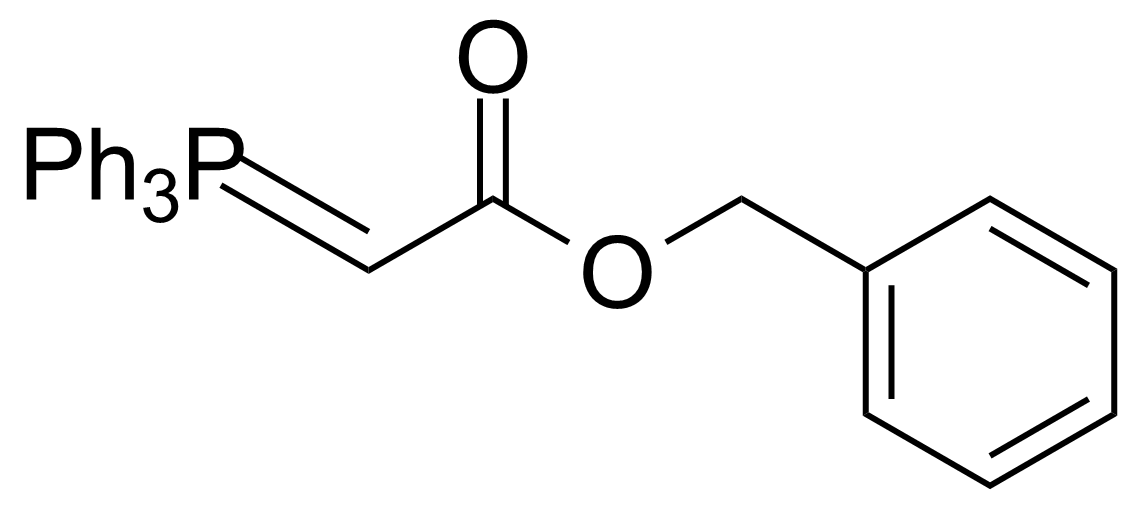 | [15097-38-8] | GEO-02511 | |
| Bis(dichlorophosphino)methane | 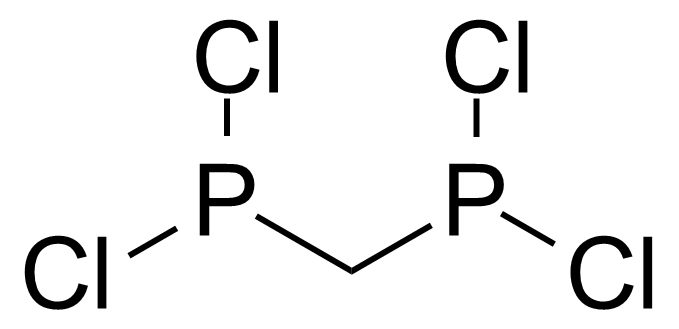 | [28240-68-8] | GEO-03420 | |
| Bis(diethylamino)chlorophosphine | 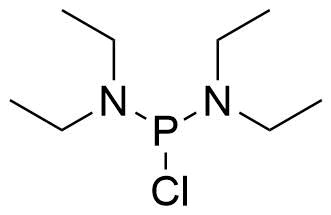 | [685-83-6] | GEO-04741 | |
| N,N-Bis(diphenylphosphino)amine | 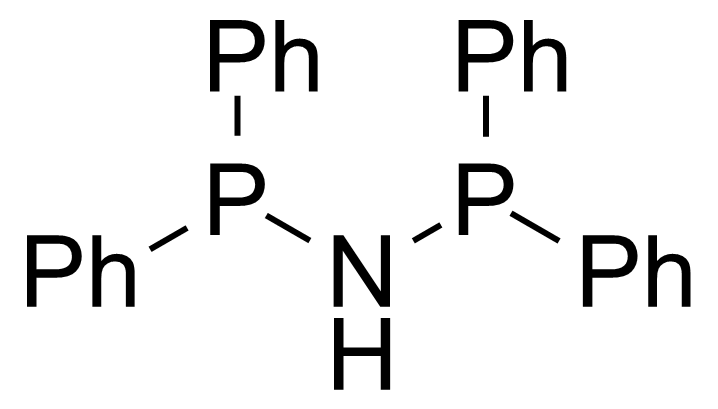 | [2960-37-4] | GEO-04010 |