Acetohydroxamic acid
Product has been discontinued, but we still have some stock remaining.
N-hydroxyacetamide
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H302 – Harmful if swallowed
H312 – Harmful in contact with skin
H315 – Causes skin irritation
H319 – Causes serious eye irritation
H332 – Harmful if inhaled
H335 – May cause respiratory irritation
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P301+312 – IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell
P302+352 – IF ON SKIN: Wash with soap and water
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Acetohydroxamic acid is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Acetohydroxamic acid in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Acetohydroxamic acid (AHA) [546-88-3] also known under trade names Lithostat (US) or Uronefrex (EU) is a compound, structurally similar to urea….
Show full descriptionGeneral description of Acetohydroxamic acid:
Acetohydroxamic acid (AHA) [546-88-3] also known under trade names Lithostat (US) or Uronefrex (EU) is a compound, structurally similar to urea. In a pure form, it is a white crystalline solid with melting point of 90-91 °C.[1] The structure of the hydroxamic acids was first brought to the attention by W. Lossen in 1869.[2] Few years later he observed, apparently by accident, that thermal decomposition of hydroxamic acid led to the isocyanate. This reaction was later named after him as Lossen rearrangement.[3] The most general method of preparation is the reaction between ethylacetate or acetic anhydride and hydroxylamine in absolute alcohol. The reaction proceeds rapidly at room temperature, particularly in the presence of an equimolecular quantity of sodium alkoxide.[4] Alternatively, AHA can be prepared from acetyl chloride and hydroxylamine with sodium carbonate in ether/water.[5]Application of Acetohydroxamic acid:
Acetohydroxamic acid is a potent urease inhibitor of bacterial urease activity, especially Helicobacter pylori.[6] It was found to be useful in treating urinary tract infections by preventing urine alkalization. Although this compound had severe side effects, such as teratogenicity, psychoneurologic and musculo-integumentary symptoms, it was approved by the FDA in 1983 to treat chronic urea-splitting urinary infections.[7] It is used, in addition to antibiotics or medical procedures, to treat chronic urea-splitting urinary infections. It can be oxidized in situ to nitrosocarbonylmethane which reacts in Diels–Alder reaction with 1,3-dienes.[5] Acetohydroxamic acid is a good chelating agent for heavy metals[8] such as copper, iron, cobalt, nickel, chromium, manganese, uranium. It has been suggested as a partitioning agent for the separation of uranium and plutonium in nuclear fuel reprocessing.[9]Product categorization (Chemical groups):
Main category: Second level: ______________________________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
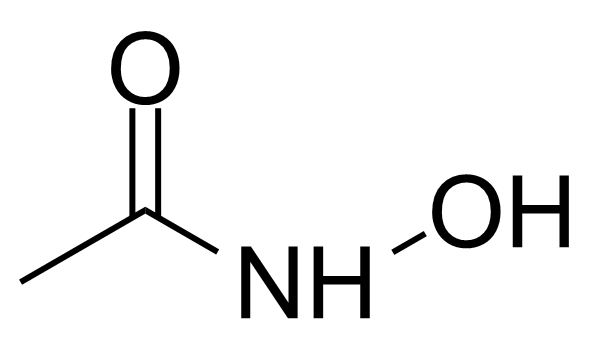
| Acetohydroxamic acid |  | [546-88-3] | GEO-00010 | |
| 1-Adamantanecarboxylic acid | 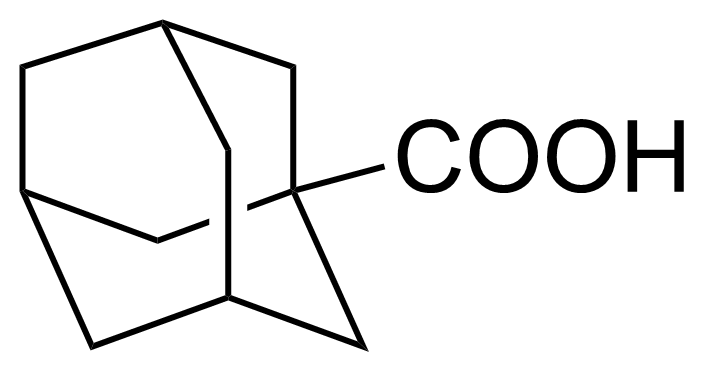 | [828-51-3] | GEO-04336 | |
| New | 4-Amino-5-nitrophthalic acid | 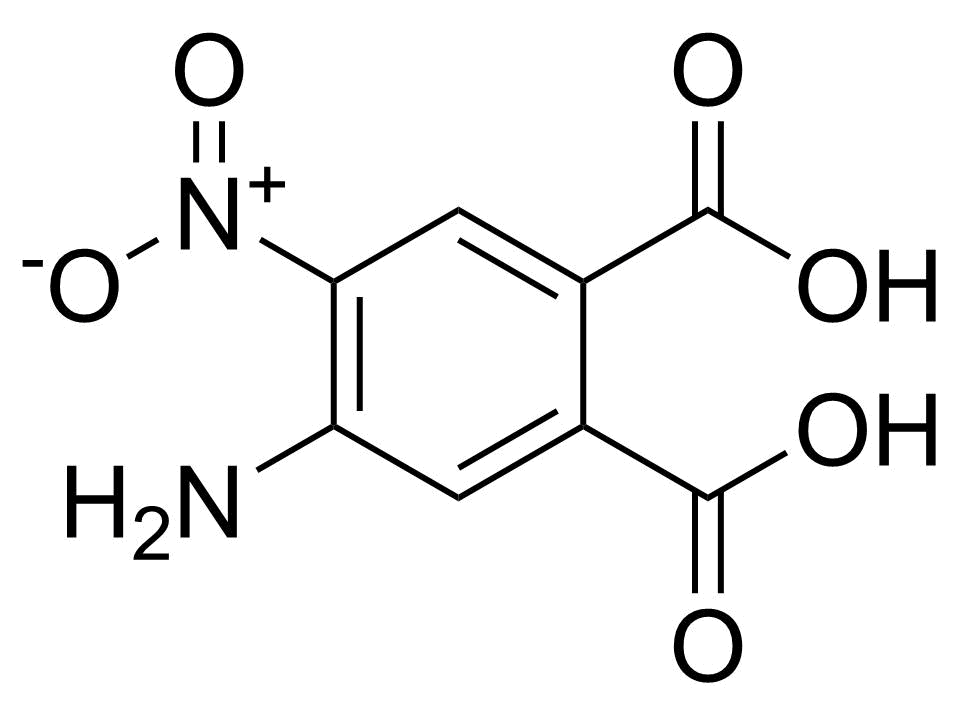 | [89939-49-1] | GEO-04810 |
| 3H-1,2-Benzodithiol-3-one 1,1-dioxide | 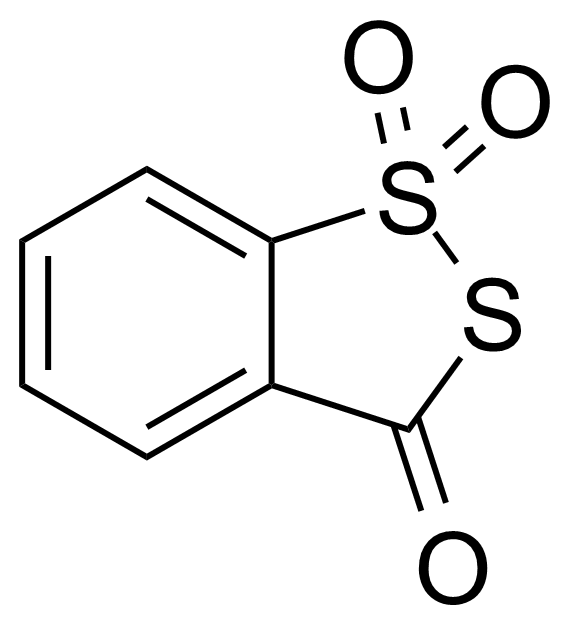 | [66304-01-6] | GEO-04361 | |
| Benzo[b]thiophene-2-carboxylic hydrazide | ![Structure of Benzo[b]thiophene-2-carboxylic hydrazide](https://georganics.sk/wp-content/uploads/2021/05/GEO-00294_Benzobthiophene-2-carboxylic_hydrazide.png) | [175135-07-6] | GEO-00294 | |
| N-Benzylmaleimide dihydrate | 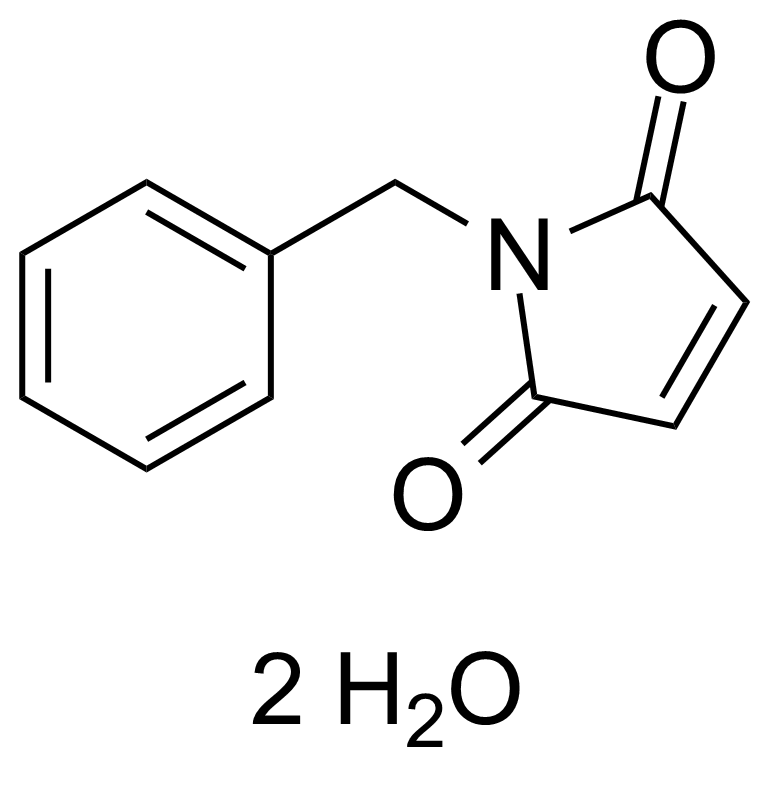 | [1631-26-1] | GEO-00303 | |
| Bromoacetic acid anhydride | 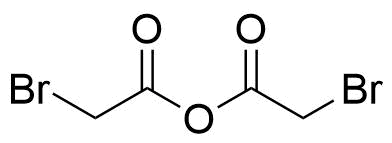 | [13094-51-4] | GEO-04577 | |
| 2-(6-Bromohexyl)isoindoline-1,3-dione | 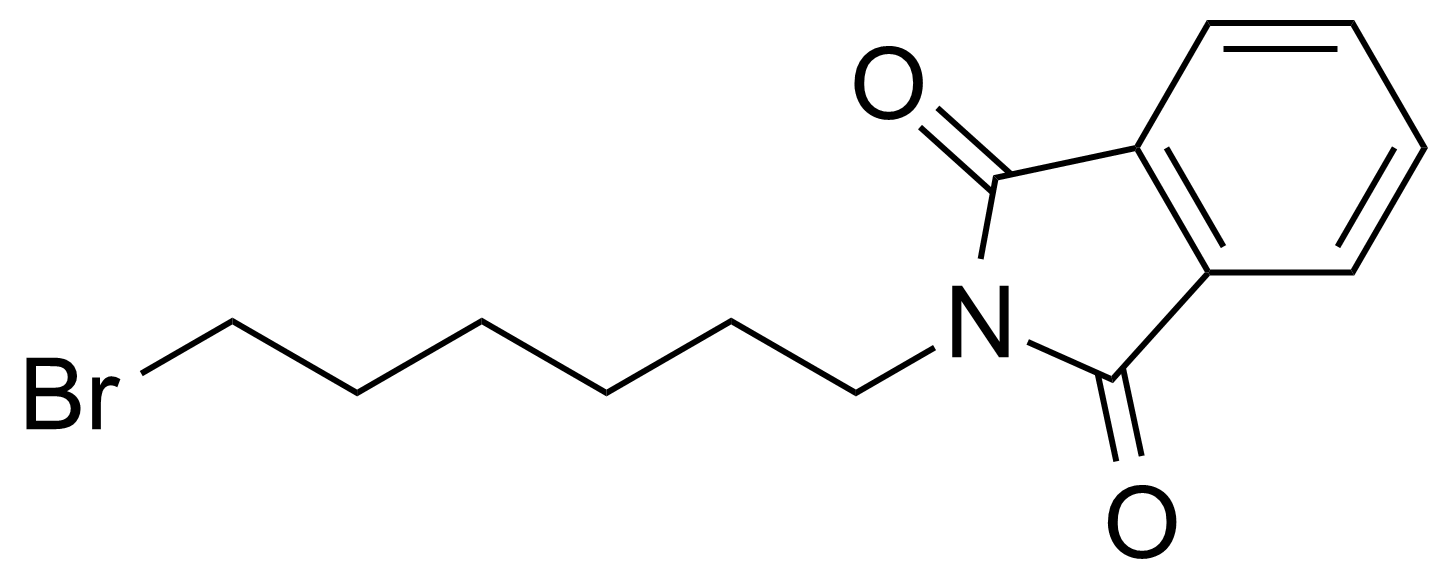 | [24566-79-8] | GEO-04399 | |
| New | 7-Bromoisoquinoline-4-carboxylic acid | 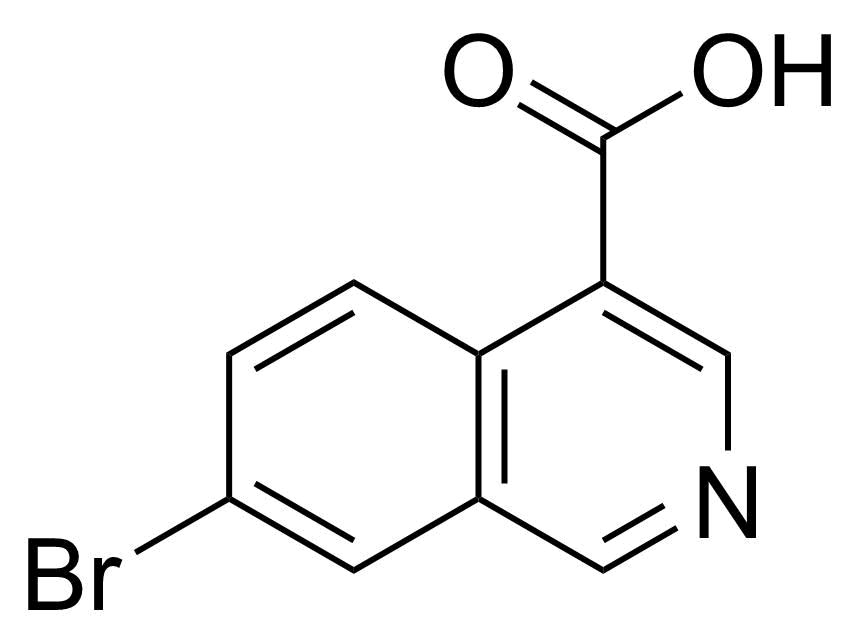 | [31009-04-8] | GEO-04828 |
| Bromomaleic anhydride |  | [5926-51-2] | GEO-00484 |