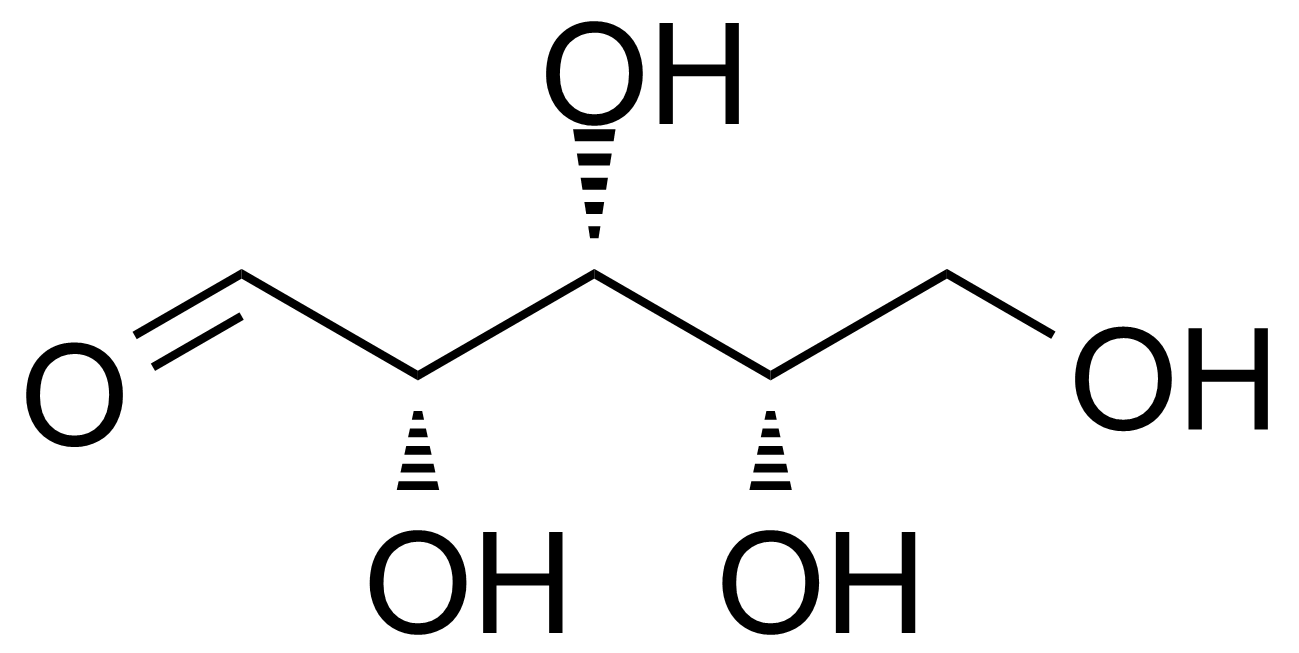
L-Xylose
CAS-Nr.[609-06-3]
G-CodeGEO-02456
EC-Nummer210-174-1
SummenformelC5H10O5
Molekulargewicht150,13
Synonyme
L-(-)-xylose ; (2S,3S,4S)-oxane-2,3,4,5-tetrol ; L-Xylopyranose
Für weitere Informationen oder eine Anfrage senden Sie uns bitte eine E-Mail oder nutzen Sie unser Kontaktformular
Regulatorische Informationen
Dieses Produkt ist nicht klassifiziert.
Produktkategorisierung
Special offer :
Zweite Ebene
Beschreibung
L-Xylose ist eine nützliche chemische Verbindung mit vielfältigen Forschungsanwendungen. Wir freuen uns, qualitativ hochwertige L-Xylose in verschiedenen Größen (für Forschungs-, Pilotmaßstabs- oder Produktionsanwendungen) von Milligramm- bis Multi-Kilogramm-Chargen anbieten zu können, sodass Sie ganz einfach die richtige Menge für Ihre Bedürfnisse auswählen können.
Vollständige Beschreibung anzeigenUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
L-Xylose [609-06-3] is a rare monosaccharide of the aldopentose type first isolated from wood, and named for it (ancient greek: ξύλον, xylon, "wood"). It is a white crystalline solid with the melting point of 144-145 °C.[1] L-Xylose can be prepared by chemical route from D-gluconolactone [2] or from D-sorbitol.[3],[4] Enzymatic isomerization of the ketosugar L-xylulose to L-xylose has been presented as an alternative for low yields chemical synthesis. The starting material, L-xylulose can be produced by oxidation of the relatively cheap polyol, xylitol, using natural bacterial isolates as whole cell catalysts.[5]
L-Xylose [609-06-3] is a rare monosaccharide of the aldopentose type first isolated from wood, and named for it (ancient greek: ξύλον, xylon, "wood"). It is a white crystalline solid with the melting point of 144-145 °C.[1] L-Xylose can be prepared by chemical route from D-gluconolactone [2] or from D-sorbitol.[3],[4] Enzymatic isomerization of the ketosugar L-xylulose to L-xylose has been presented as an alternative for low yields chemical synthesis. The starting material, L-xylulose can be produced by oxidation of the relatively cheap polyol, xylitol, using natural bacterial isolates as whole cell catalysts.[5]
Application of Furoin:
L-xylose is used in organic synthesis as a chiral building block. It was used for the synthesis of L-ascorbic acid.[6] Novel L-xylose derivatives have recently been reported to act as inhibitors of urinary glucose reabsorption, which suggests that they may find use in diabetes treatment.[7] Polyhydroxypyrrolidines derived from l-xylose have shown antitumor and anti-HIV properties and act as potent α- and β-glucosidase inhibitors, which also is of relevance for the development of diabetes drugs.[8]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________[1] T. G. Bonner, E. J. Bourne, S. E. Harwood, D. Lewis J. Chem. Soc. 1965, 121. doi:10.1039/JR9650000121 [2] W. B. Yang, S. S. Patil, C. H. Tsai, C. H. Lin, J. M. Fang Tetrahedron 2002, 58 (2), 253. doi:10.1016/S0040-4020(01)01146-2 [3] E. Dimant, M. Banay J. Org. Chem. 1960, 25 (3), 475. doi:10.1021/jo01073a621 [4] R. C. Hockett Production of l-xylose 1952, HEINZ M WUEST, US2584129A. [5] A. Usvalampi, O. Turunen, J. Valjakka, O. Pastinen, M. Leisola, A. Nyyssölä Enzyme. Microb. Technol. 2012, 50 (1), 71. doi:10.1016/j.enzmictec.2011.09.009 [6] L. L. Salomon, J. J. Burns, C. G. King J. Am. Chem. Soc. 1952, 74 (20), 5161. doi:10.1021/ja01140a051 [7] N. C. Goodwin, R. Mabon, B. A. Harrison, M. K. Shadoan, Z. Y. Almstead, Y. Xie, J. Healy, L. M. Buhring, C. M. DaCosta, J. Bardenhagen, F. Mseeh, Q. Liu, A. Nouraldeen, A. G. E. Wilson, S. D. Kimball, D. R. Powell, D. B. Rawlins J. Med. Chem. 2009, 52 (20), 6201. doi:10.1021/jm900951n [8] J. B. Behr, G. Guillerm Tetrahedron Lett. 2007, 48 (13), 2369. doi:10.1016/j.tetlet.2007.01.125
Ähnliche Produkte
| Produktname | Struktur | CAS-Nr. | G-Code | |
|---|---|---|---|---|
| 1-Acenaphthenol | 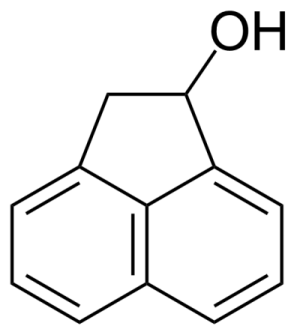 | [6306-07-6] | GEO-00001 | |
| 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 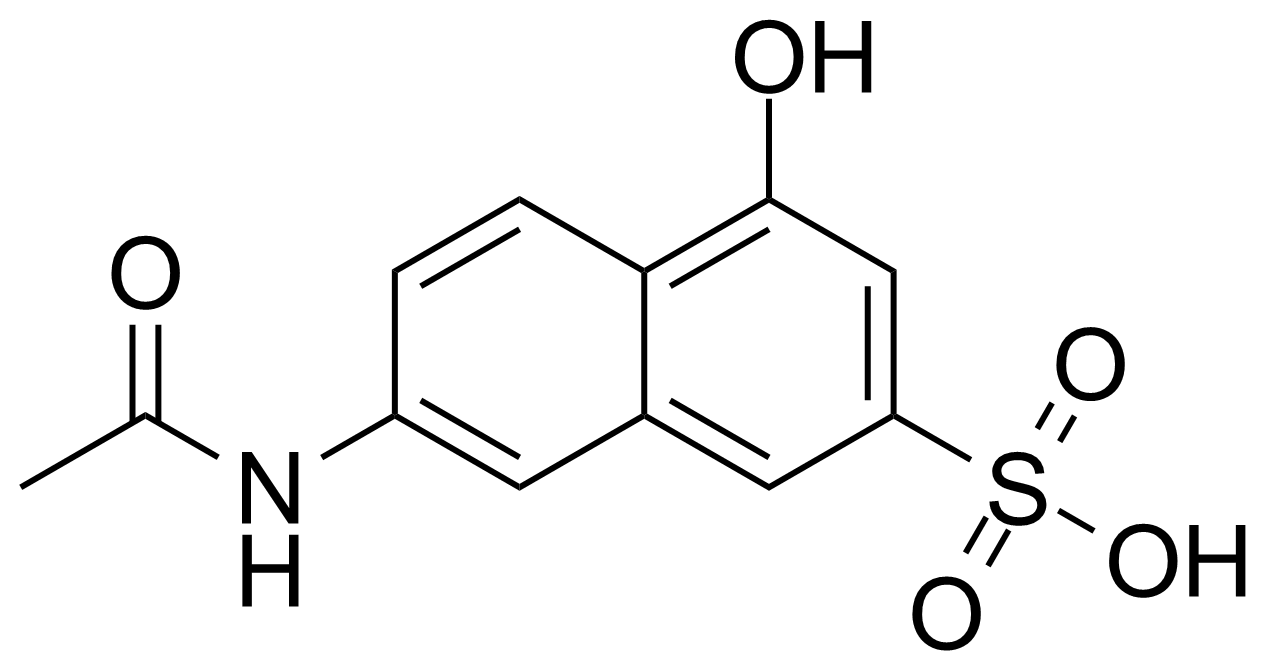 | [6334-97-0] | GEO-04013 | |
| 1-Adamantanemethanol | 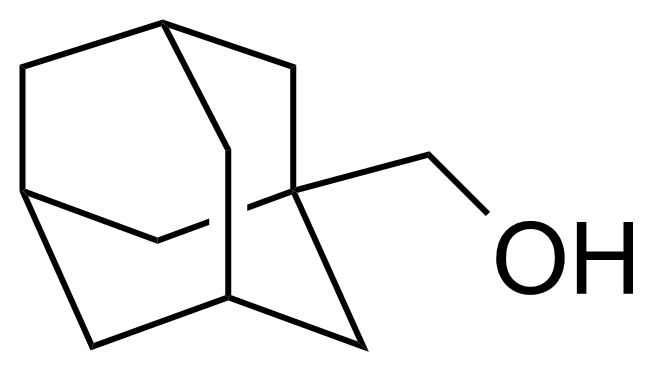 | [770-71-8] | GEO-04333 | |
| beta-D-Allopyranose | 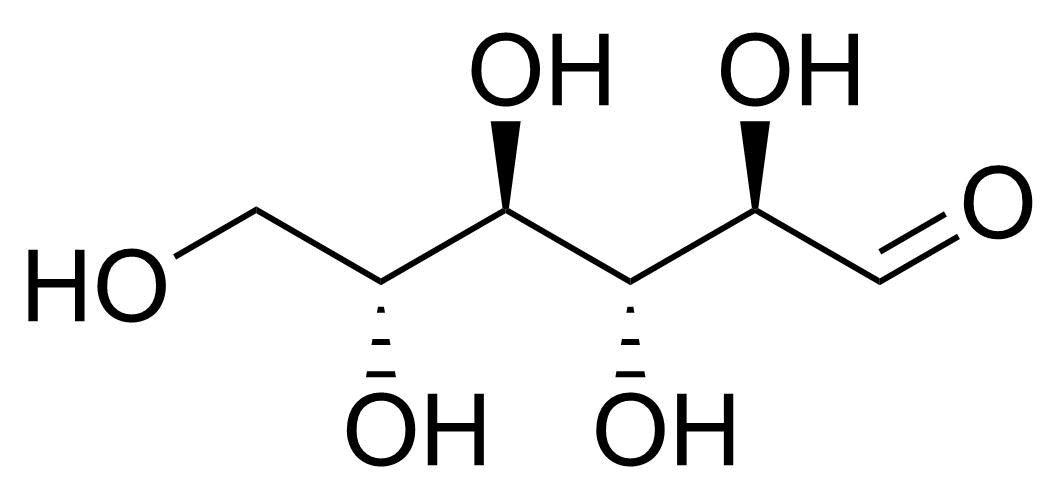 | [7283-09-2] | GEO-04660 | |
| D-Allose | 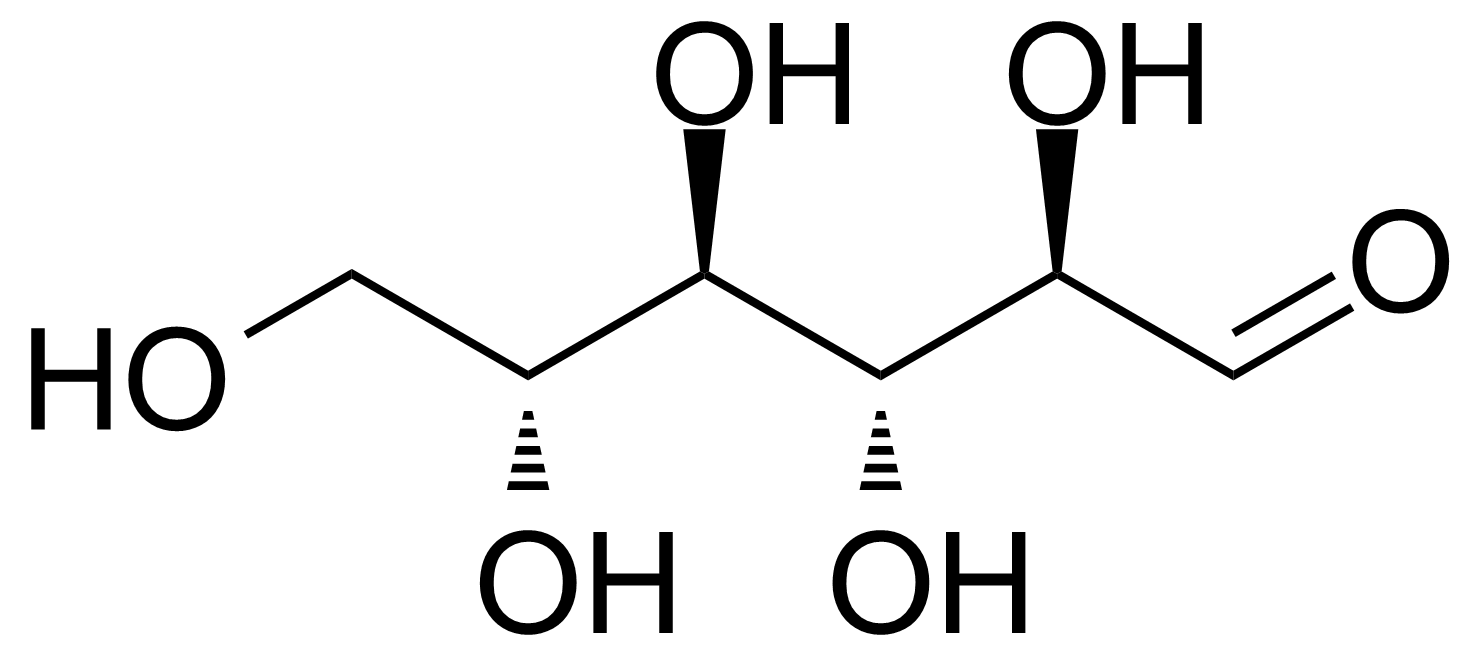 | [2595-97-3] | GEO-00057 | |
| L-Allose | 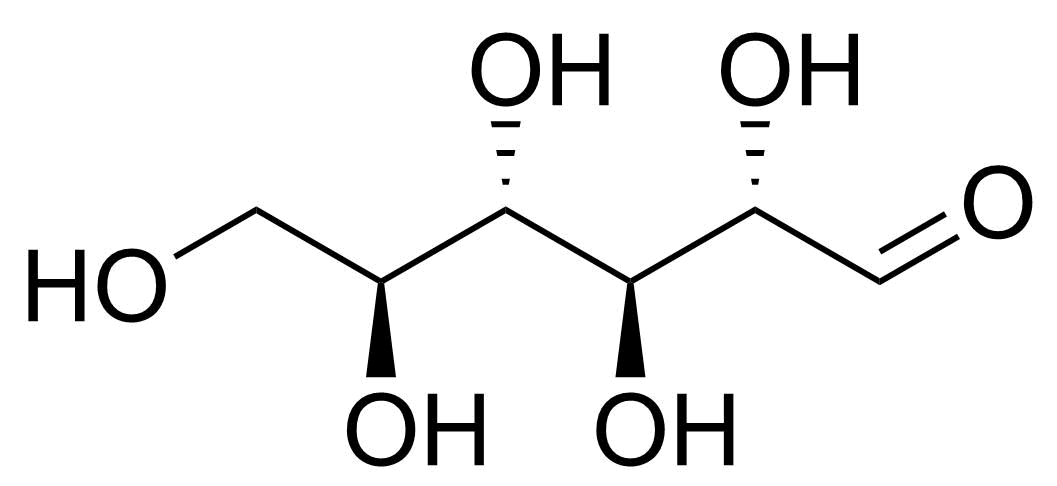 | [39392-62-6] | GEO-04661 | |
| 2-(Allyloxy)phenol | 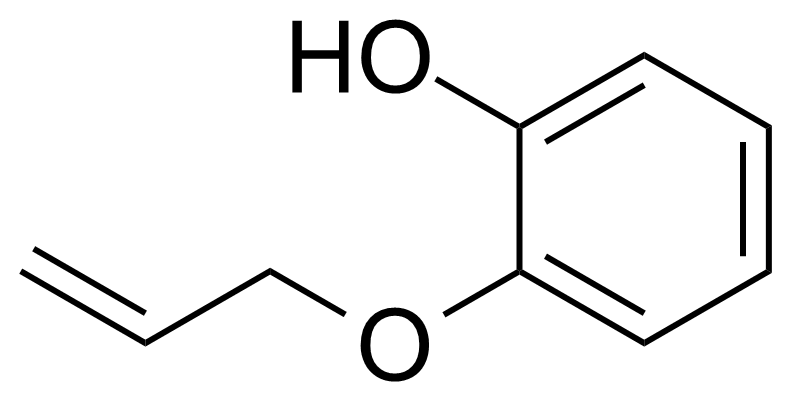 | [1126-20-1] | GEO-04471 | |
| D-Altrose | 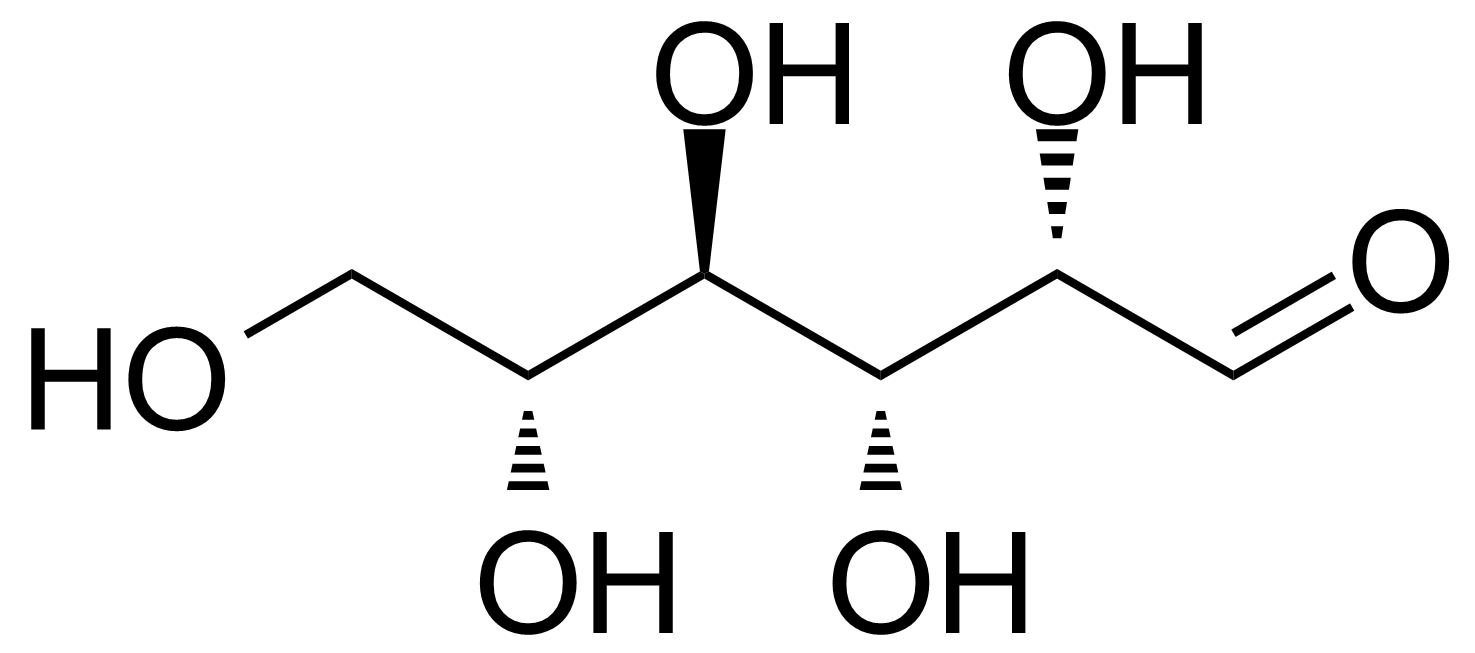 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 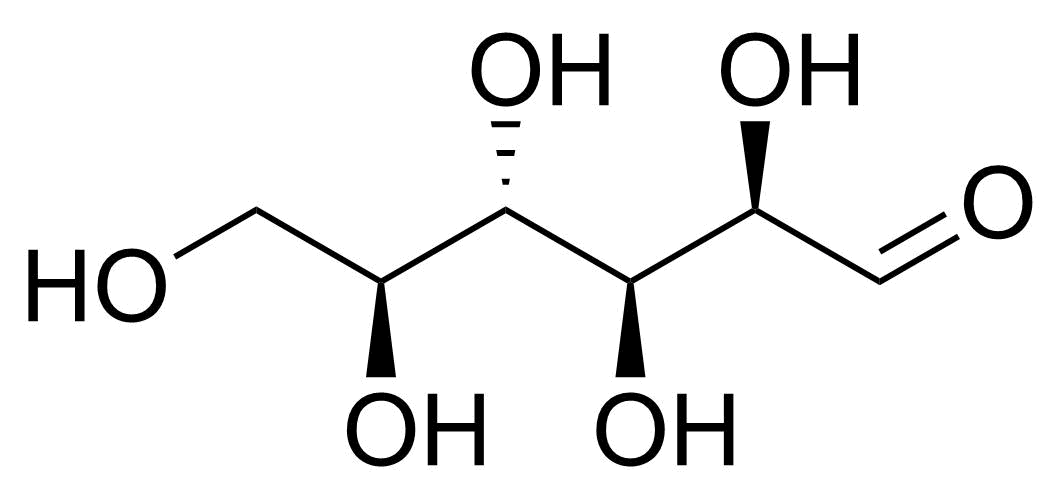 | [1949-88-8] | GEO-04662 | |
| (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 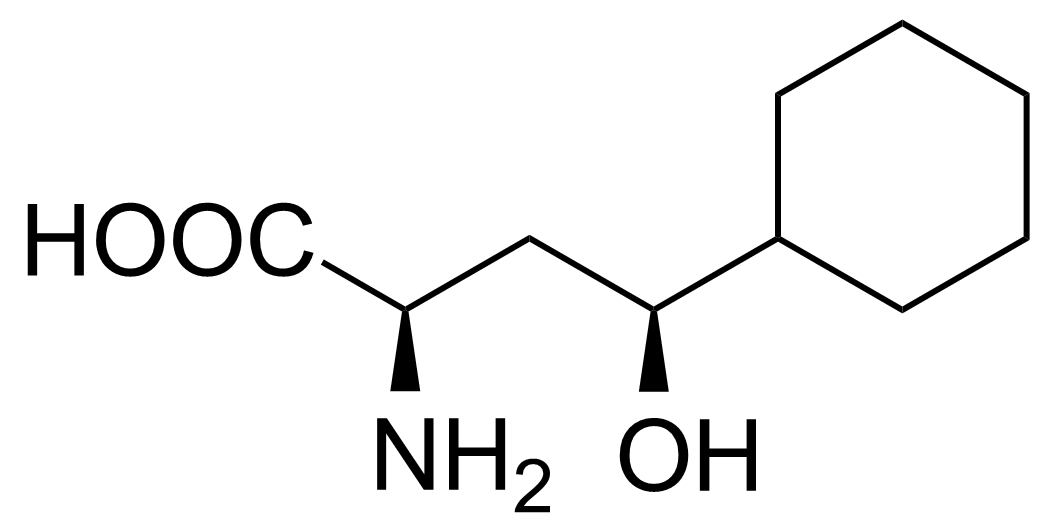 | [] | GEO-02717 |