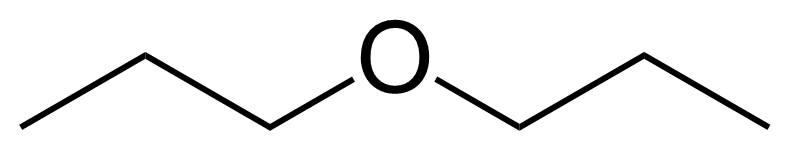
Propyl ether
Di-n-propyl ether ; Dipropyl ether ; 1-propoxypropane ; 1,1'-Oxybis[propane] ; 4-Oxaheptane ; Dipropyl oxide
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information


EUH019 – May form explosive peroxides
EUH066 – Repeated exposure may cause skin dryness or cracking
H225 – Highly flammable liquid and vapour
H336 – May cause drowsiness or dizziness
P210 – Keep away from heat/sparks/open flames/hot surfaces – No smoking:
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P301+330+331 – IF SWALLOWED: Rinse mouth. Do NOT induce vomiting
P303+361+353 – IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower
P304+340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Propyl ether is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Propyl ether in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Propyl ether, as other ethers, it can be used as a solvent. It is also a versatile reagent for the construction of C-C bonds…
Show full descriptionApplication of Propyl ether:
As other ethers, it can be used as a solvent. It is also a versatile reagent for the construction of C-C bonds via sp3 α-C-H activation reaction.[4] It can be used in tert-butyl hydroperoxide (TBHP)-promoted tandem acylation/cyclization of 1,6-dienes under catalyst- and base-free conditions with a key step being cleavage of C(sp3)-H and C(sp3)-O bond of ether.[5] Propyl ether can be selectively oxidized at α-position to appropriate ester with sodium hypochlorite catalysed by ruthenium.[6]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| New | 5-Acetyl-2-amino-4-(4-methoxyphenyl)-6-methyl-4H-pyran-3-carbonitrile | 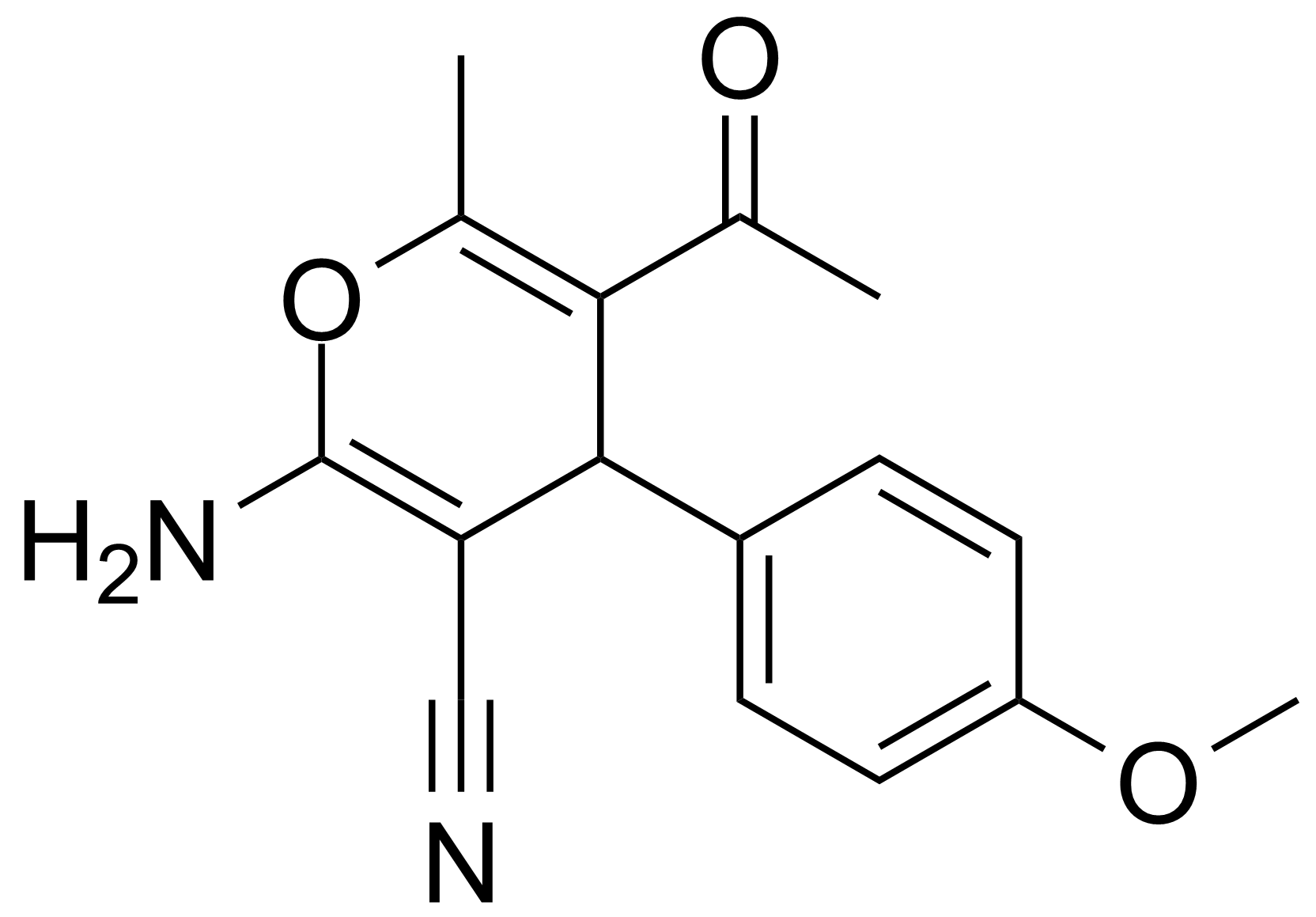 | [105263-07-8] | GEO-00017 |
| New | 2-(Allyloxy)phenol | 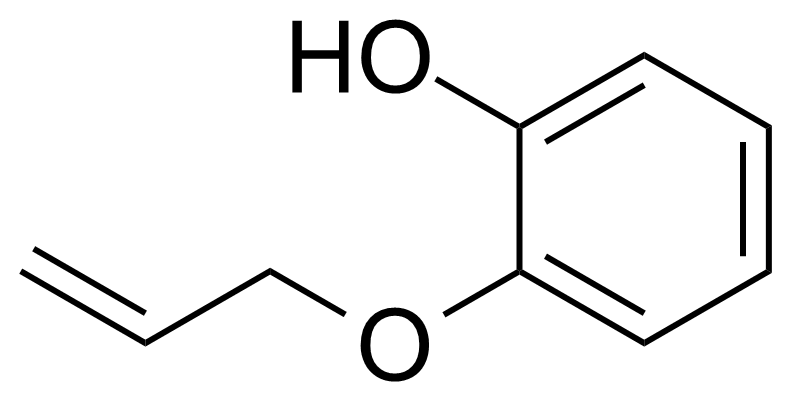 | [1126-20-1] | GEO-04471 |
| New | 4-Allyloxy 2,2,6,6-tetramethylpiperidine | 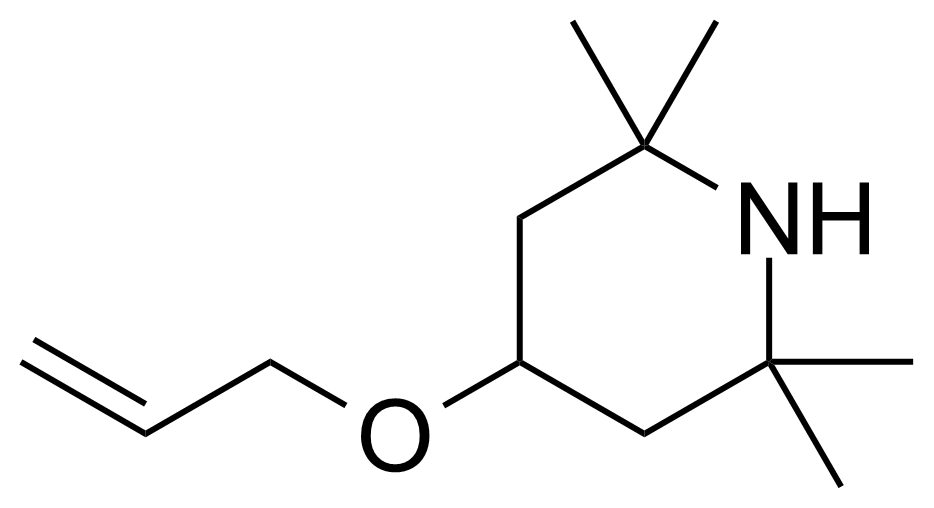 | [43224-75-5] | GEO-03985 |
| New | 4-Amino-3-methoxybenzoic acid | 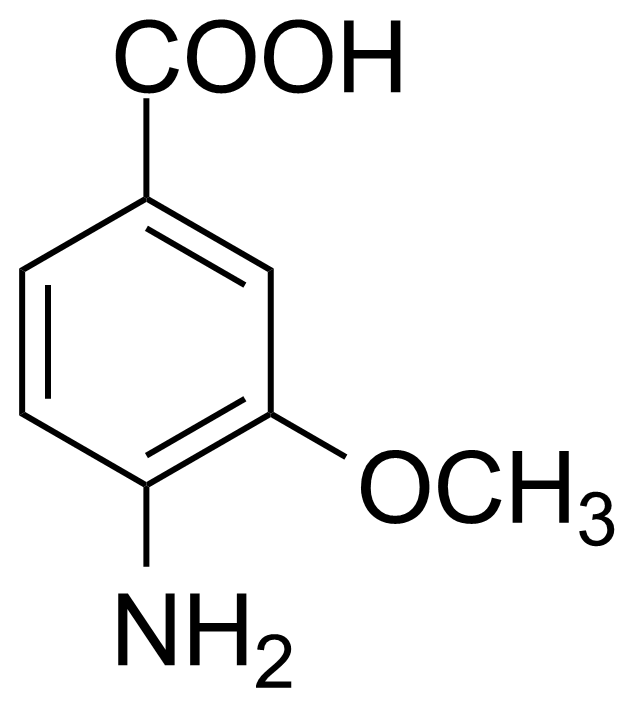 | [2486-69-3] | GEO-00154 |
| New | 2-Amino-4-methoxyphenol | 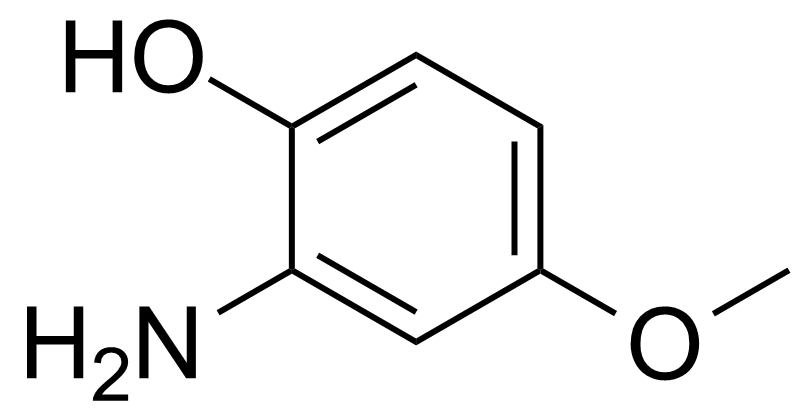 | [20734-76-3] | GEO-03978 |
| New | 1,5-Anhydro-3,4,6-tri-O-benzyl-2-deoxy-D-arabinohex-1-enitol | 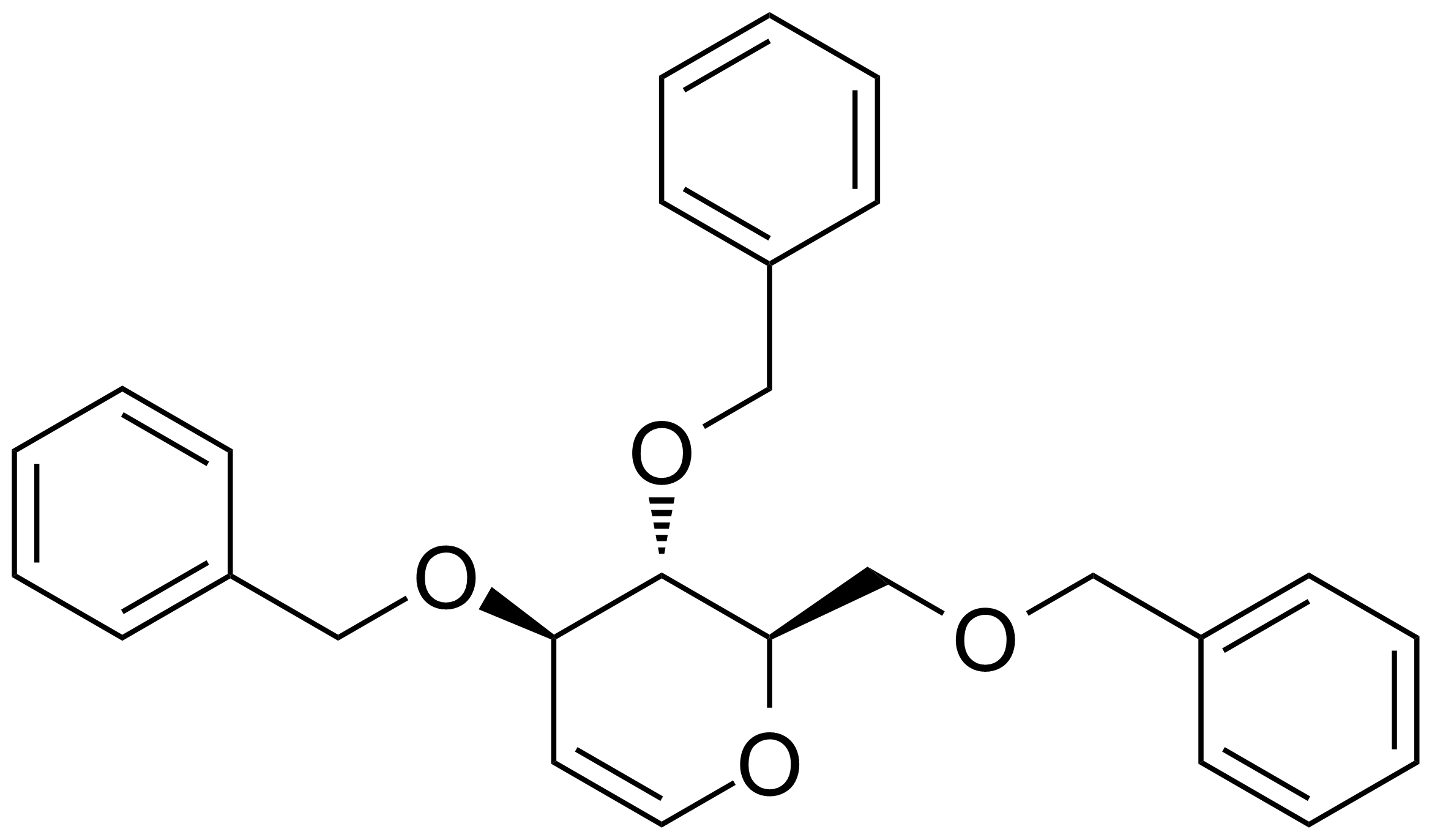 | [55628-54-1] | GEO-00234 |
| New | (R,S)-Atenolol | 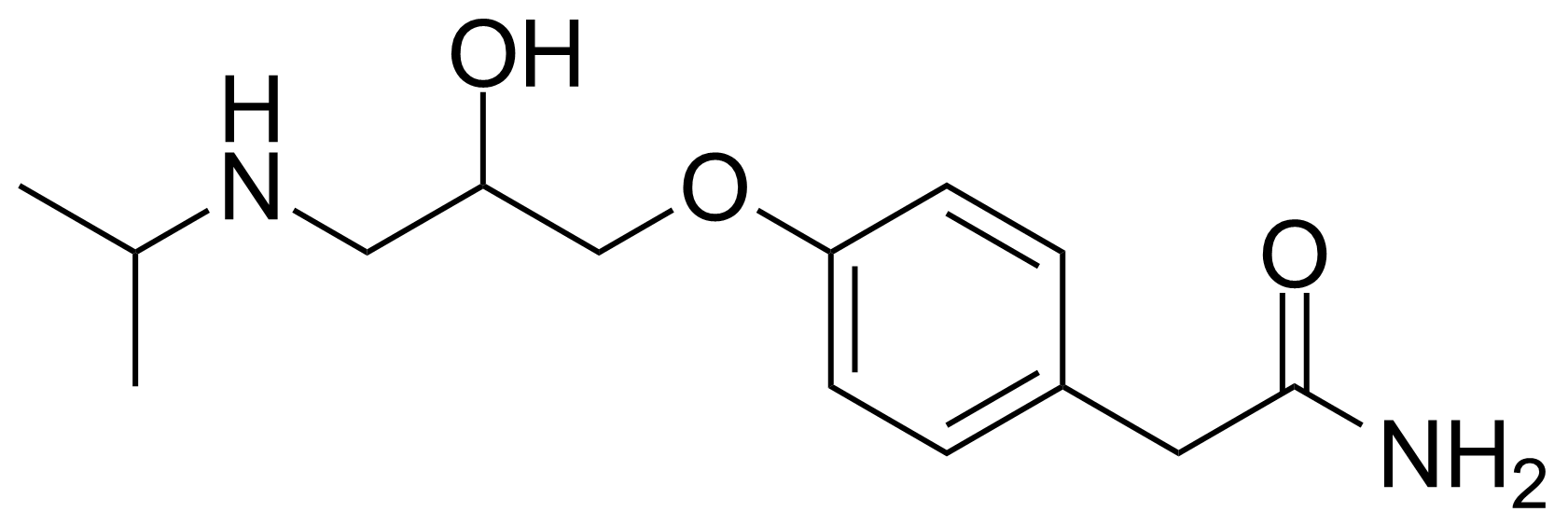 | [29122-68-7] | GEO-03413 |
| New | (R)-(+)-Atenolol | 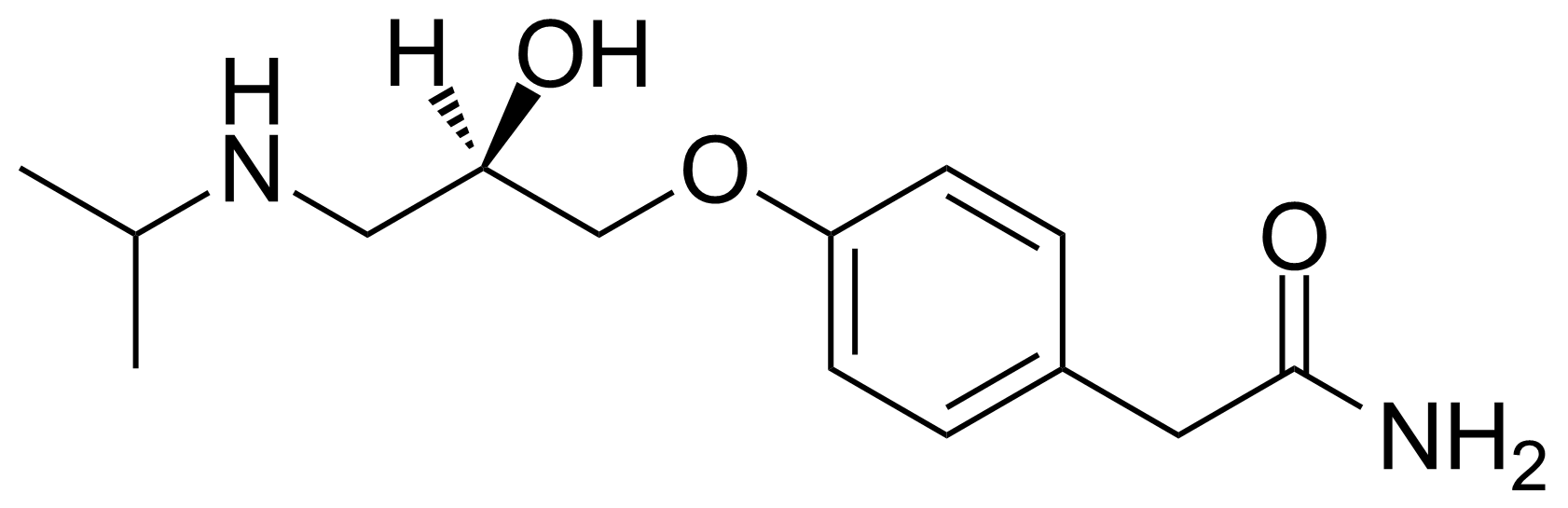 | [56715-13-0] | GEO-02767 |
| New | (S)-(-)-Atenolol | 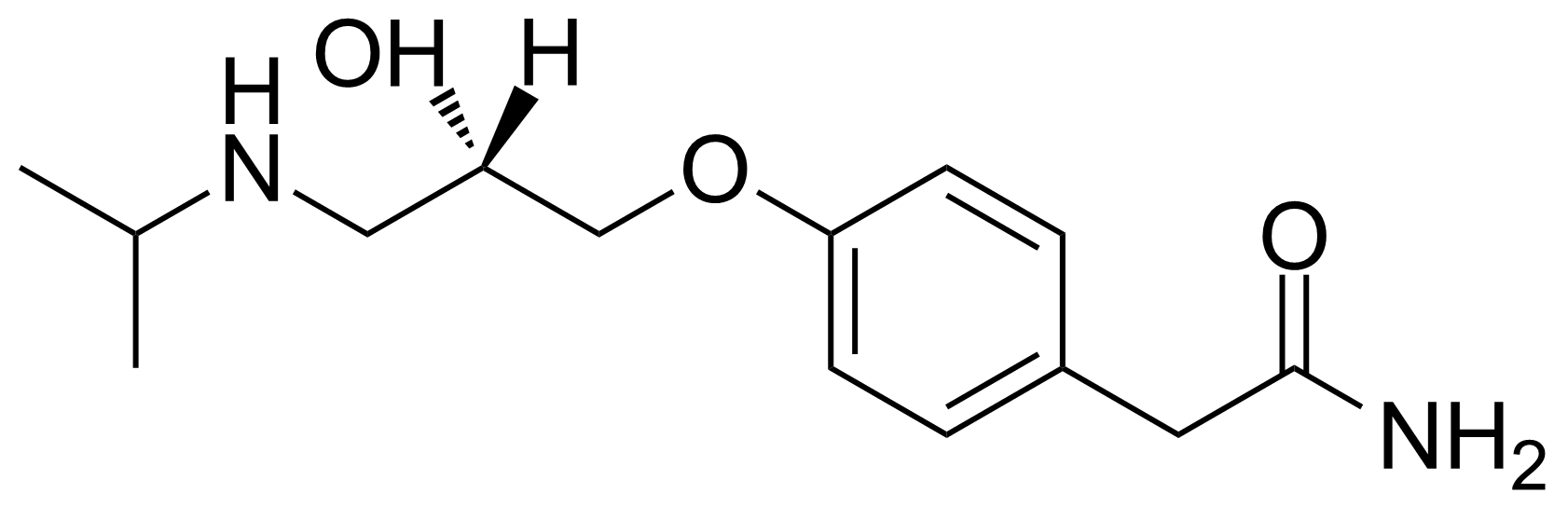 | [93379-54-5] | GEO-02804 |
| New | 4-(2-(Benzofuran-2-yl)quinolin-4-yl)morpholine | 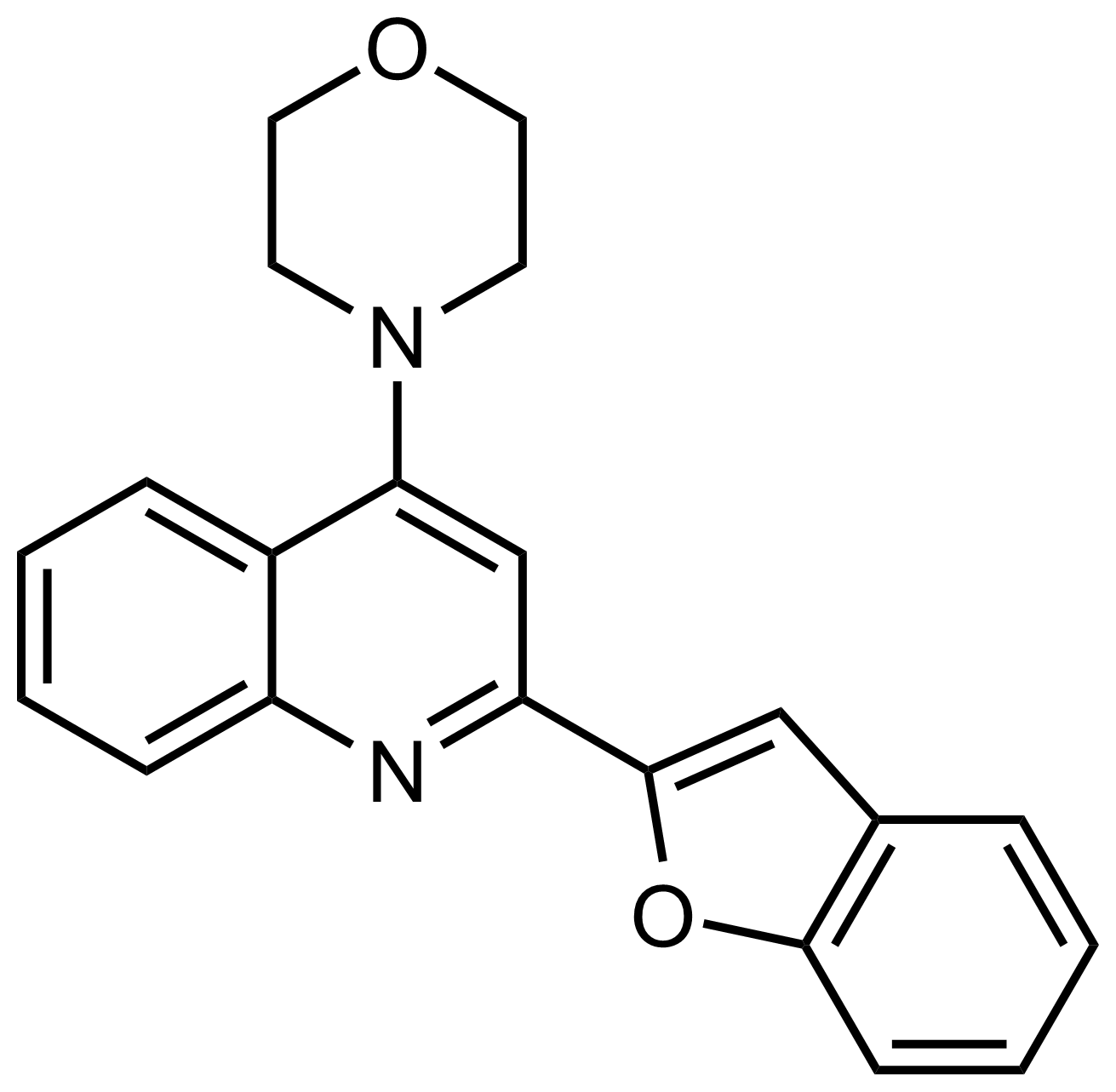 | N/A | GEO-03294 |