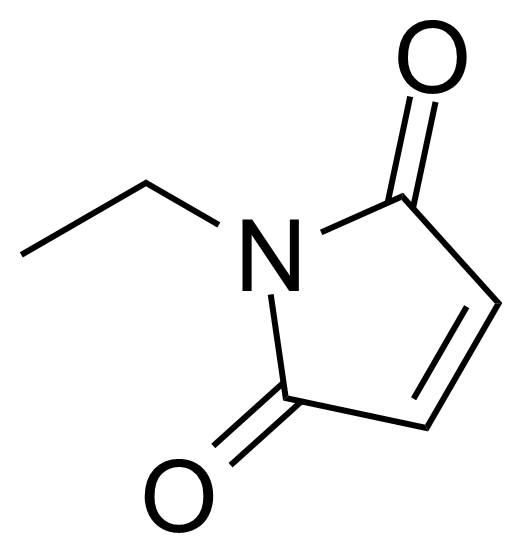
N-Ethylmaleimide
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information
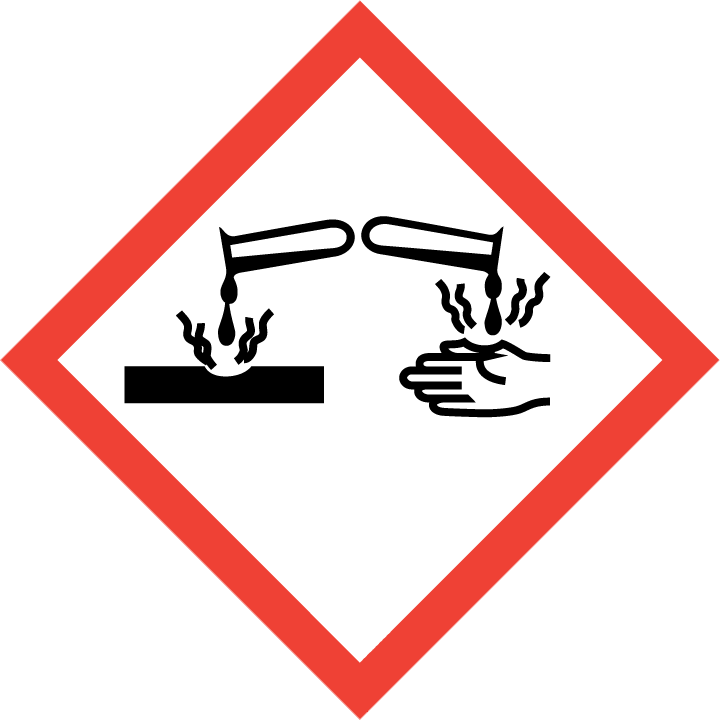

H300 – Fatal if swallowed.
H311 – Toxic in contact with skin.
H314 – Causes severe skin burns and eye damage.
H317 – May cause an allergic skin reaction.
H318 – Causes serious eye damage.
P260 – Do not breathe dust/fume/gas/mist/vapours/spray.
P270 – Do no eat, drink or smoke when using this product.
P280 – Wear protective gloves/protective clothing/eye protection/face protection.
P303+P361+P353 – IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
P304+P340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Product categorization
Description
N-Ethylmaleimide is a useful chemical compound with a variety of research applications. We are pleased to offer high quality N-Ethylmaleimide in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Show full descriptionApplication of N-Ethylmaleimide:
It is a Michael acceptor that is reactive toward nucleophiles such as thiols therefore it is commonly used to modify cysteine residues in proteins and peptides. NEM has been widely used to probe the functional role of thiol groups in enzymology. NEM is an irreversible inhibitor of all cysteine peptidases.[3] N-Ethylmaleimide was used by Arthur Kornberg (Nobel Prize in Physiology or Medicine 1959) and colleagues to knock out DNA polymerase III to compare its activity to that of DNA polymerase I.[4] It blocks vesicular transport. In lysis buffers, 20 to 25 mM of NEM is used to inhibit de-sumoylation of proteins for Western Blot analysis. NEM inactivates endogenous deubiquitinating enzymes and specifically inhibits phosphate transport in mitochondria.[5] It was used in synthesis of novel chromone-maleimide hybrids as anti-inflammatory agents for acute lung injury.[6] NEM can be used as a valuable reagent for intramolecular 1,3-dipolar cycloadditions[7], oxidative [3 + 2] cycloadditions with benzylamines[8] or Diels-Alder reactions.[9]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| Acetohydroxamic acid | 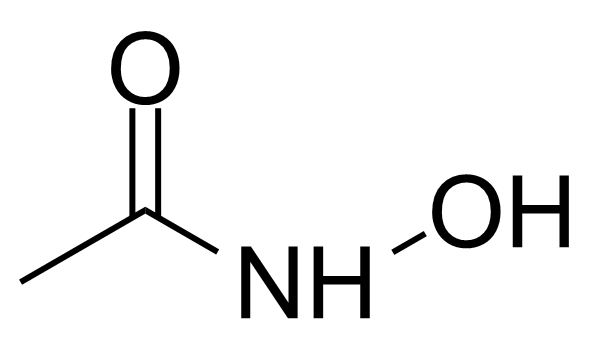 | [546-88-3] | GEO-00010 | |
| 1-Adamantanecarboxylic acid | 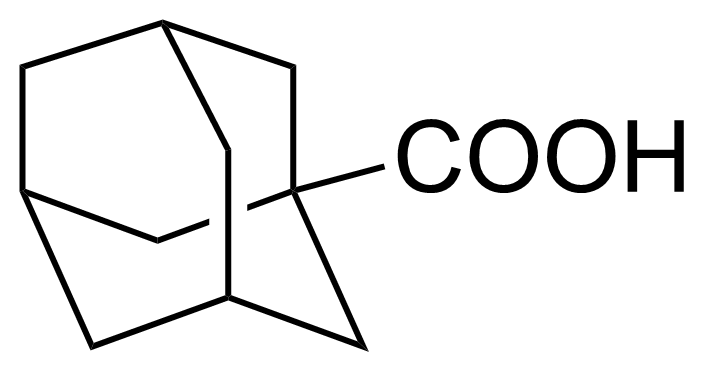 | [828-51-3] | GEO-04336 | |
| New | 4-Amino-5-nitrophthalic acid | 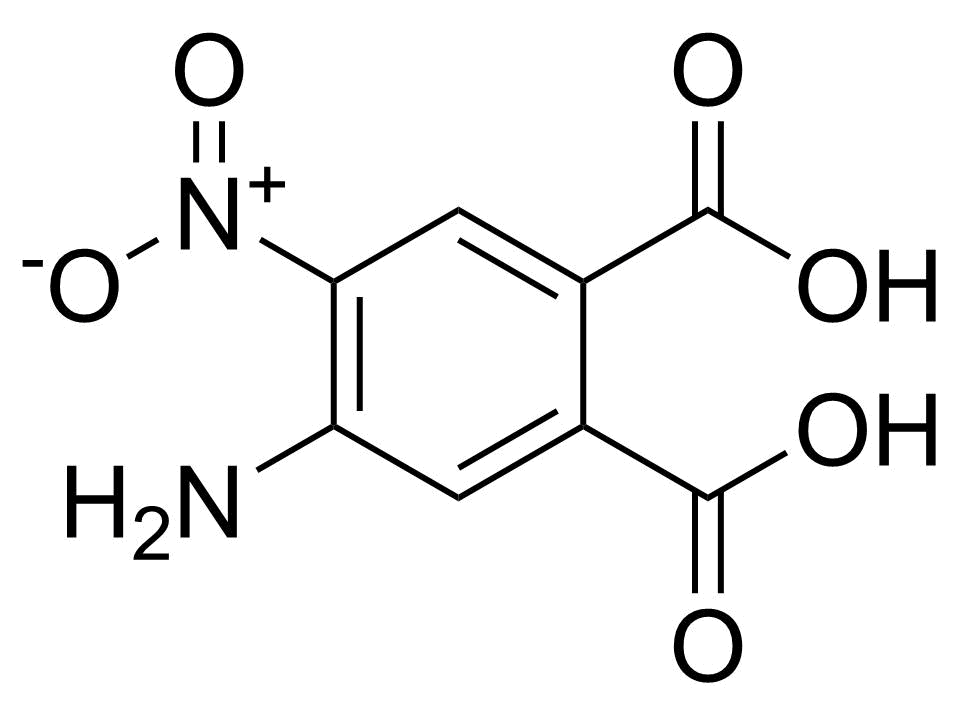 | [89939-49-1] | GEO-04810 |
| 3H-1,2-Benzodithiol-3-one 1,1-dioxide | 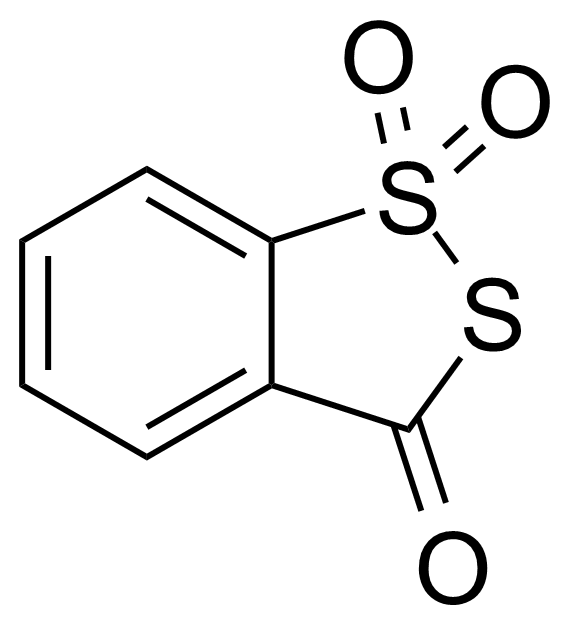 | [66304-01-6] | GEO-04361 | |
| Benzo[b]thiophene-2-carboxylic hydrazide | ![Structure of Benzo[b]thiophene-2-carboxylic hydrazide](https://georganics.sk/wp-content/uploads/2021/05/GEO-00294_Benzobthiophene-2-carboxylic_hydrazide.png) | [175135-07-6] | GEO-00294 | |
| N-Benzylmaleimide dihydrate | 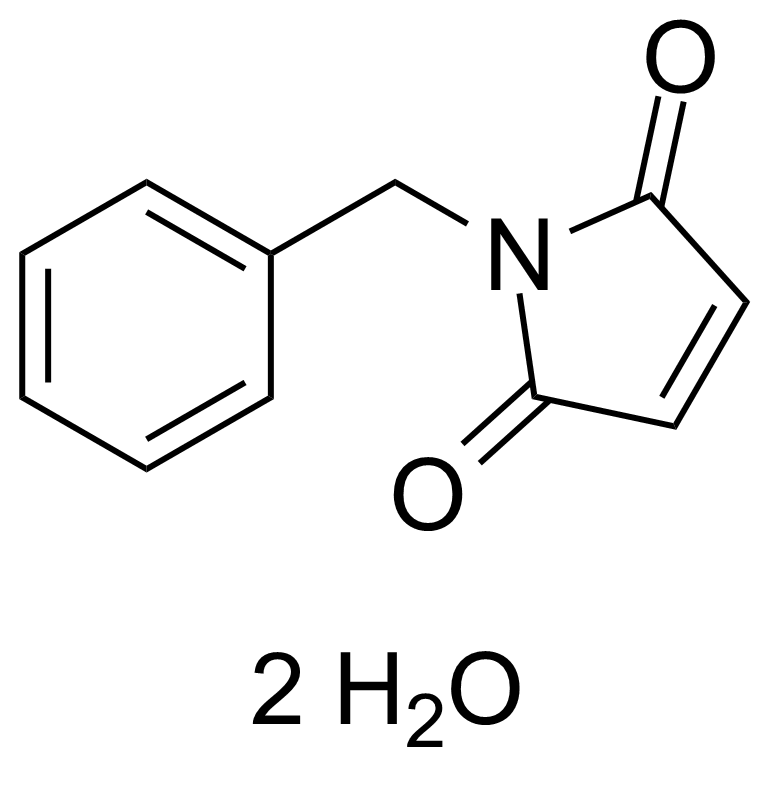 | [1631-26-1] | GEO-00303 | |
| Bromoacetic acid anhydride | 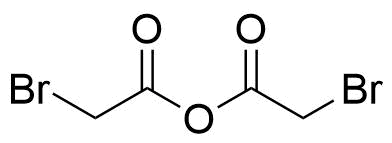 | [13094-51-4] | GEO-04577 | |
| 2-(6-Bromohexyl)isoindoline-1,3-dione | 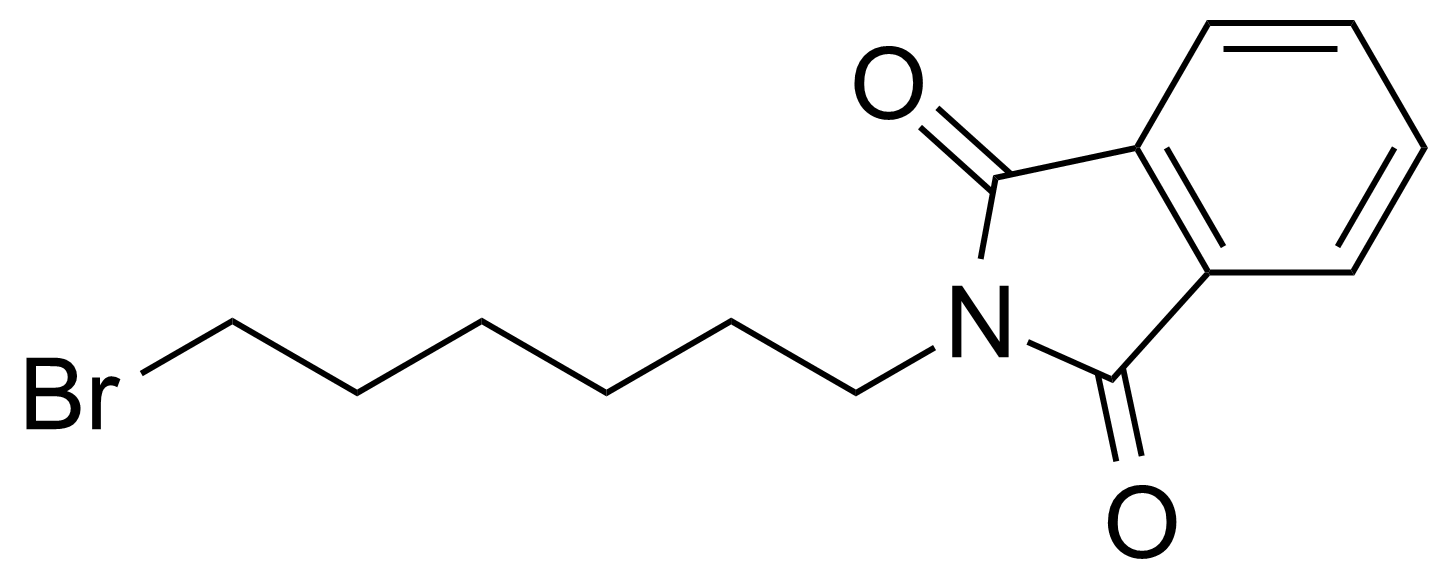 | [24566-79-8] | GEO-04399 | |
| New | 7-Bromoisoquinoline-4-carboxylic acid | 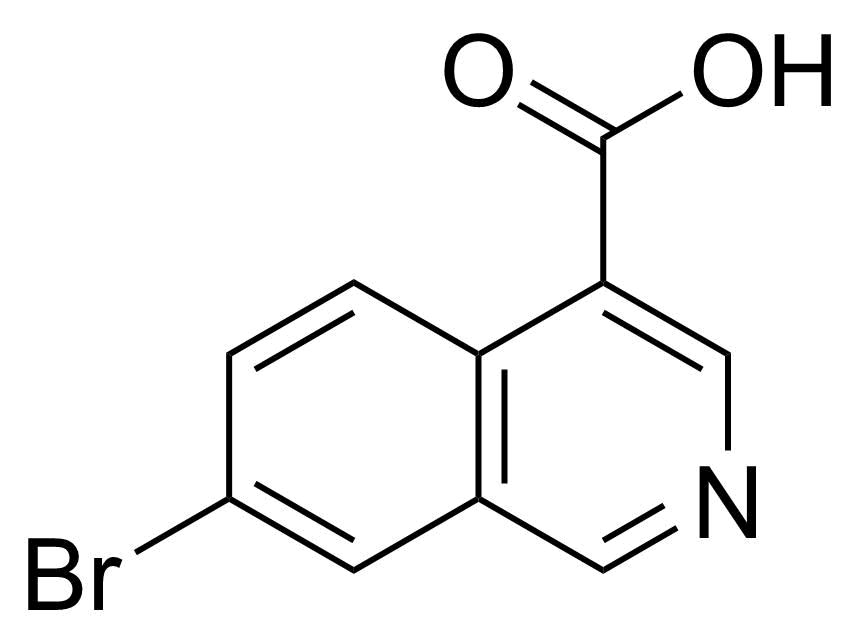 | [31009-04-8] | GEO-04828 |
| Bromomaleic anhydride | 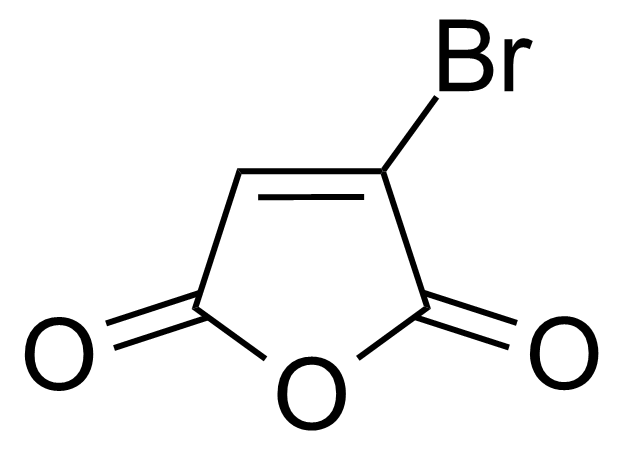 | [5926-51-2] | GEO-00484 |