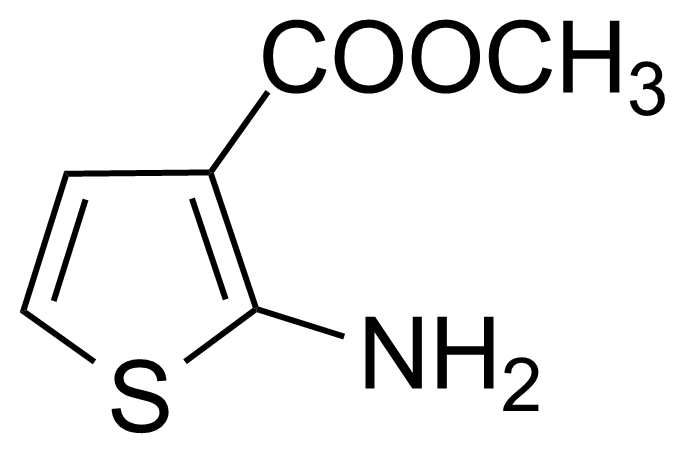
Methyl 2-amino-3-thiophenecarboxylate
methyl 2-amino-3-thenoate ; methyl 2-aminothiophene-3-carboxylate ; 2-Amino-3-carbomethoxythiophene ; 2-Amino-3-methoxycarbonylthiophene ; 2-Amino-3-thiophenecarboxylic acid methylester
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H315 – Causes skin irritation
H319 – Causes serious eye irritation
H335 – May cause respiratory irritation
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P302+352 – IF ON SKIN: Wash with soap and water
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Methyl 2-amino-3-thiophenecarboxylate is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Methyl 2-amino-3-thiophenecarboxylate in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Show full descriptionPreparation of Methyl 2-amino-3-thiophenecarboxylate:
It can be prepared by modifed Gewald reaction[2] using methyl cyanoacetate and 1,4-dithiane-2,5-diol in the presence of triethylamine in methanol. [3]Application of Methyl 2-amino-3-thiophenecarboxylate:
2-Aminothiophene derivatives are important five-membered building blocks in organic synthesis. Urea derivatives of 2-amino-3-carbomethoxythiophene exhibited promising in vitro cytotoxicity against a human cancer cell lines as an effective binders of ribonucleotidereductase protein.[4] It was used as a building block in synthesis of various thienopyrimidine derivatives which are interesting structural element in development of pharmaceutical compounds.[5] Among others, these heterocycles have been used as part of kinase inhibitors to regulate dysfunctional cell signalling in cancer cells,[6] as calcium receptor antagonists,[7] transglutaminase inhibitors,[8] as peptidase IV inhibitors[9] and against hepatitis C virus infections.[10]Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| New | 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-beta-D-glucopyranosyl azide | 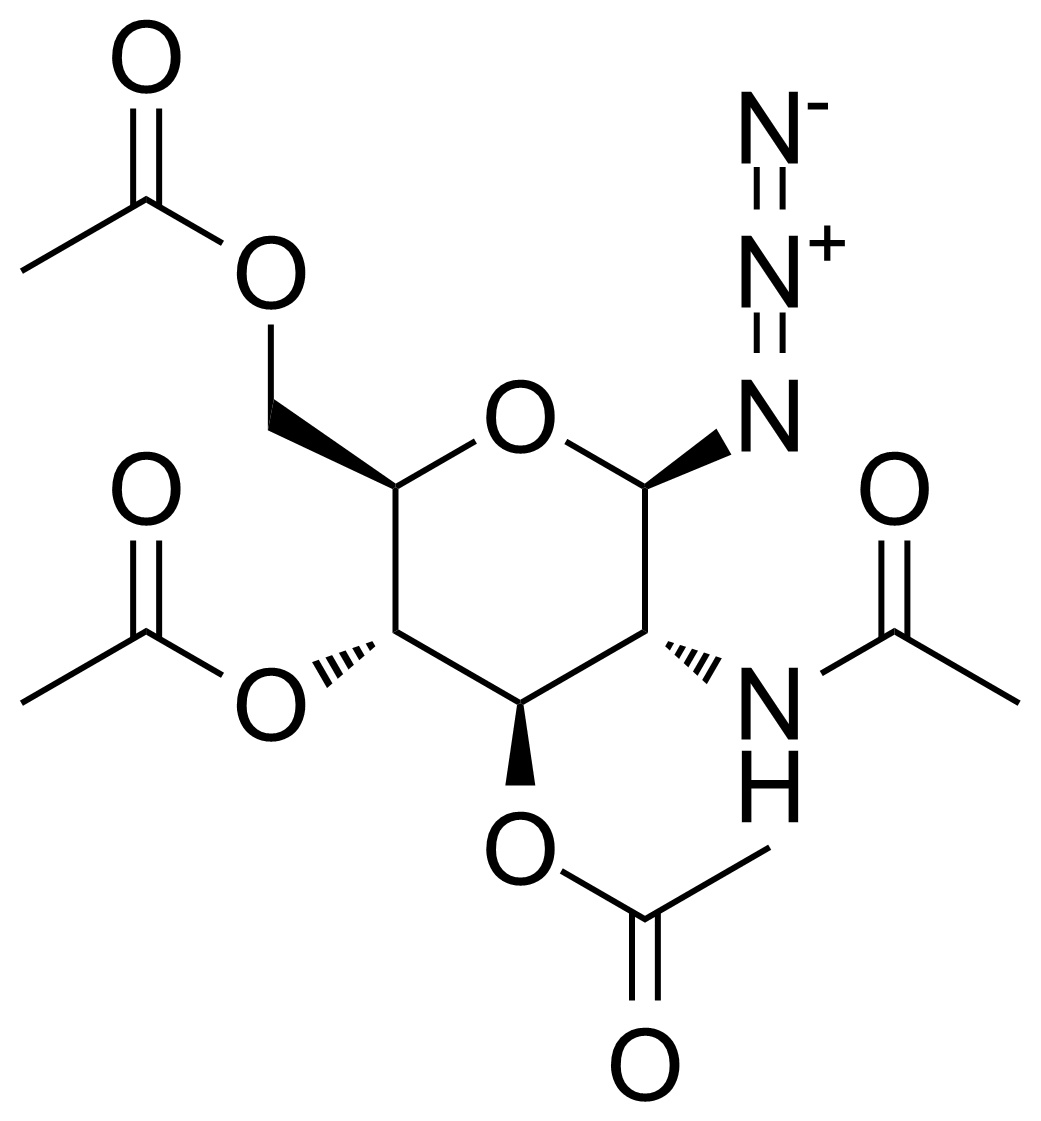 | [6205-69-2] | GEO-03200 |
| New | 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-alpha-D-glucopyranosyl chloride | 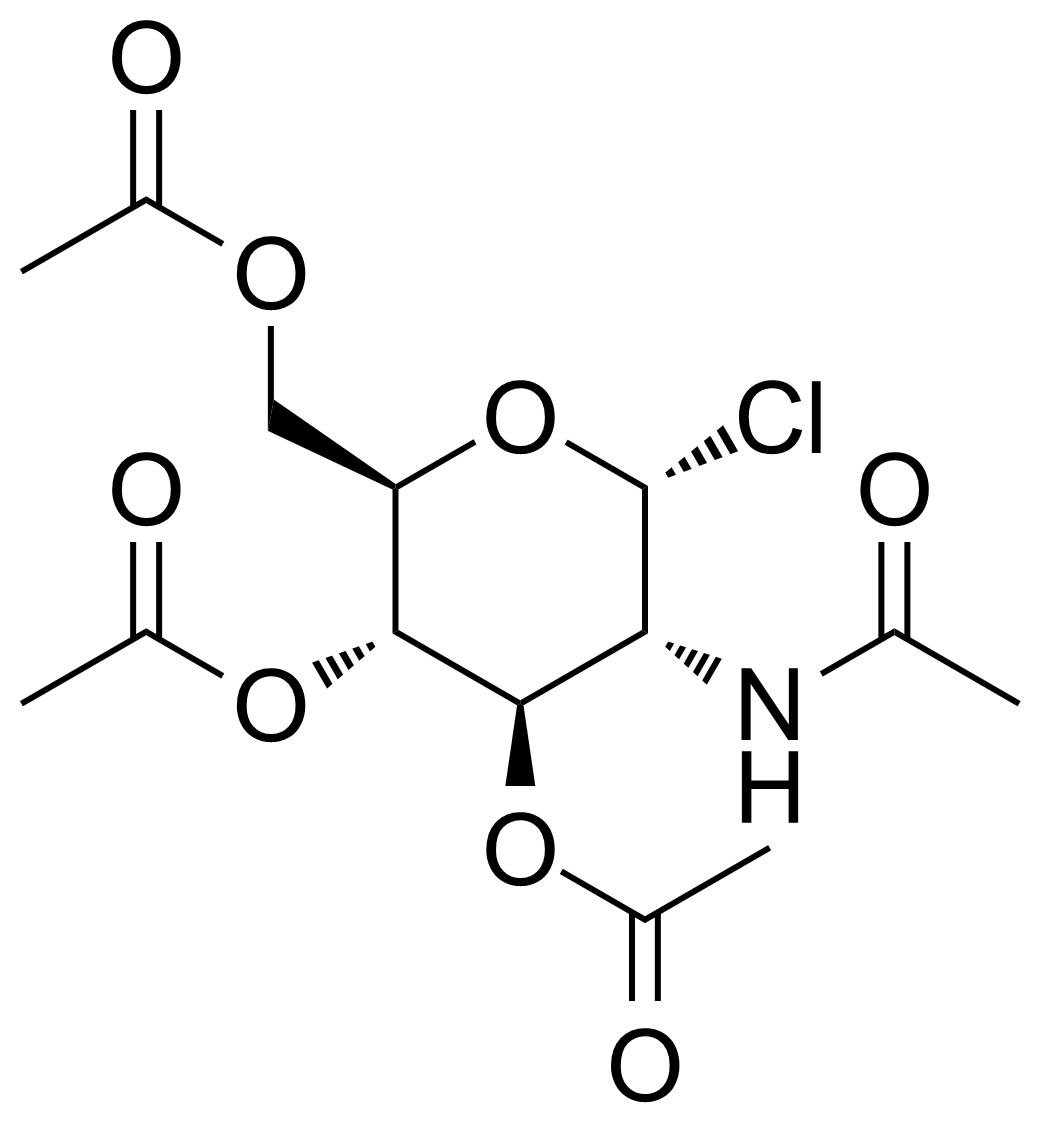 | [3068-34-6] | GEO-03374 |
| New | 2-Acetamido-1,3,4,6-tetra-O-acetyl-2-deoxy-beta-D-glucopyranose | 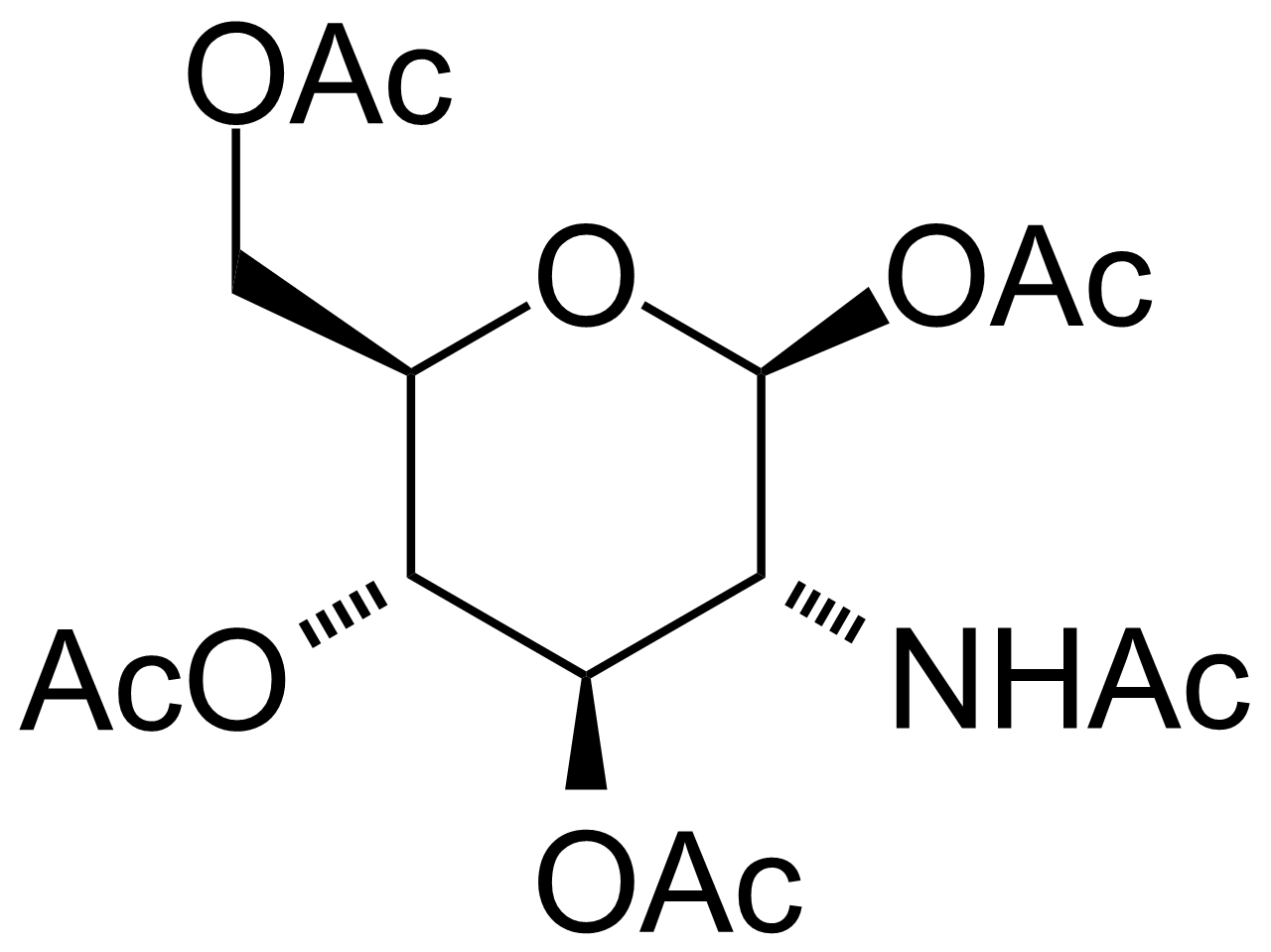 | [7772-79-4] | GEO-02610 |
| New | Acetochloro-beta-D-glucose | 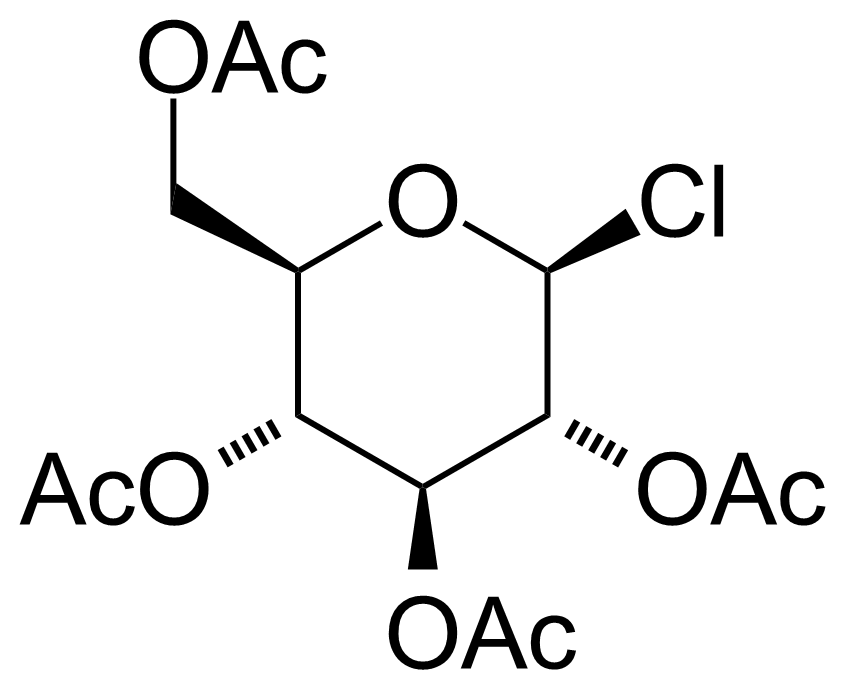 | [4451-36-9] | GEO-00008 |
| 5-Acetoxymethyl-2-furaldehyde | 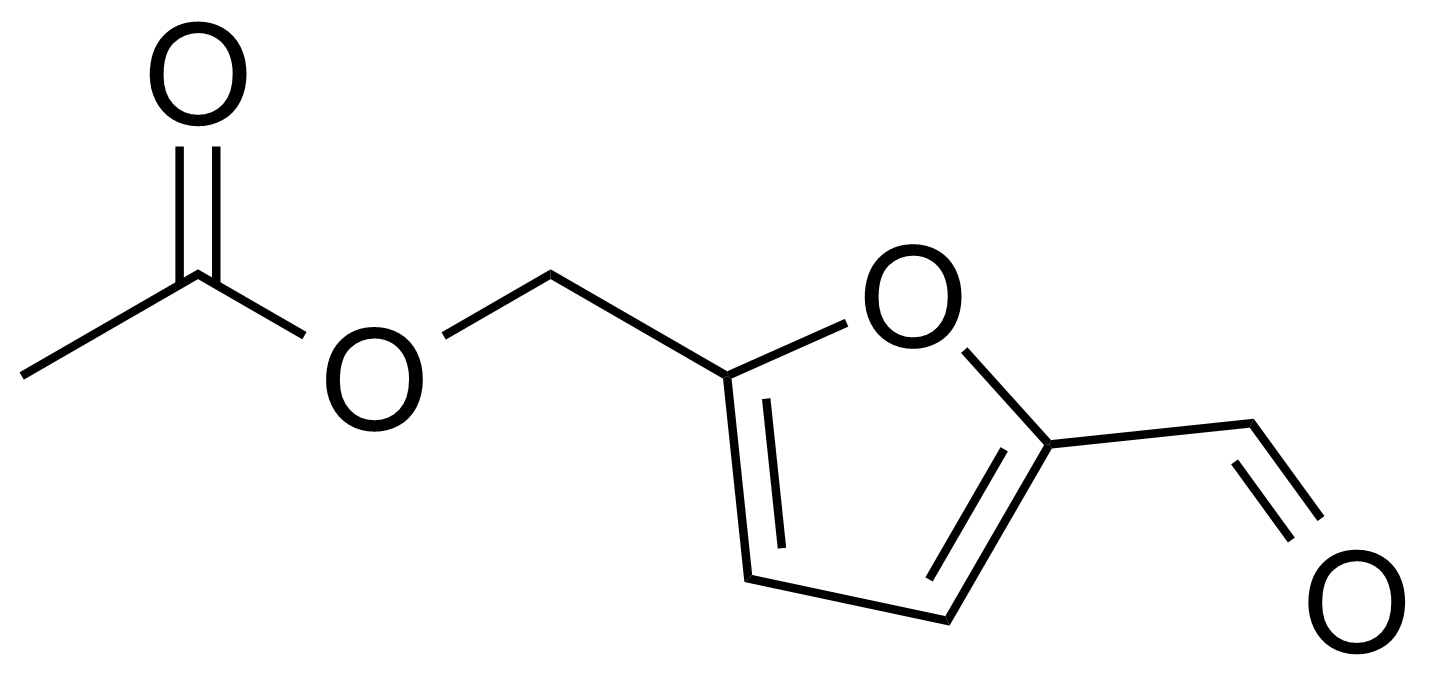 | [10551-58-3] | GEO-00013 | |
| New | 2-Acetyl-4-methyl-4-pentenoic acid methyl ester | 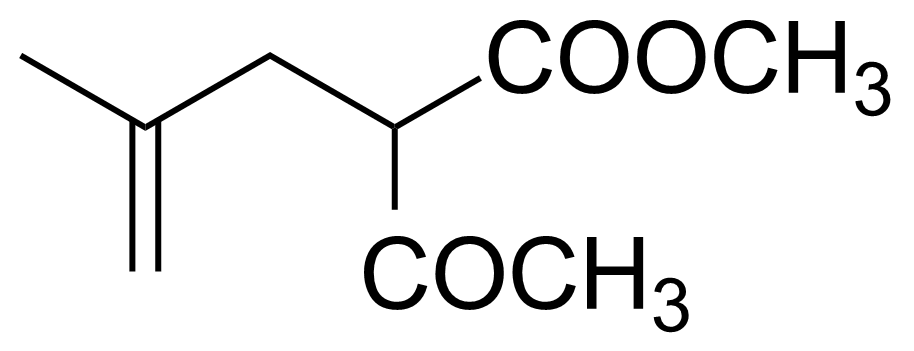 | [20962-71-4] | GEO-03240 |
| New | 5-(Acetyloxy)-2(5H)-furanone | 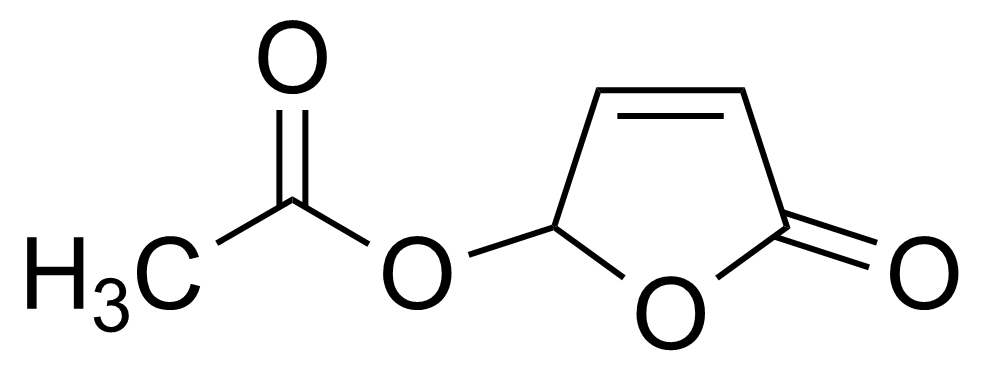 | [122952-20-9] | GEO-00007 |
| New | 1-Adamantyl methacrylate | 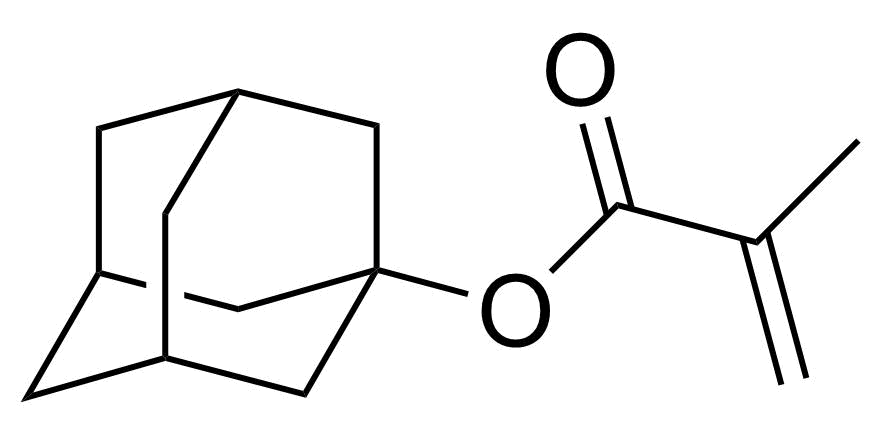 | [16887-36-8] | GEO-04510 |
| New | Adipic acid monoethyl ester | 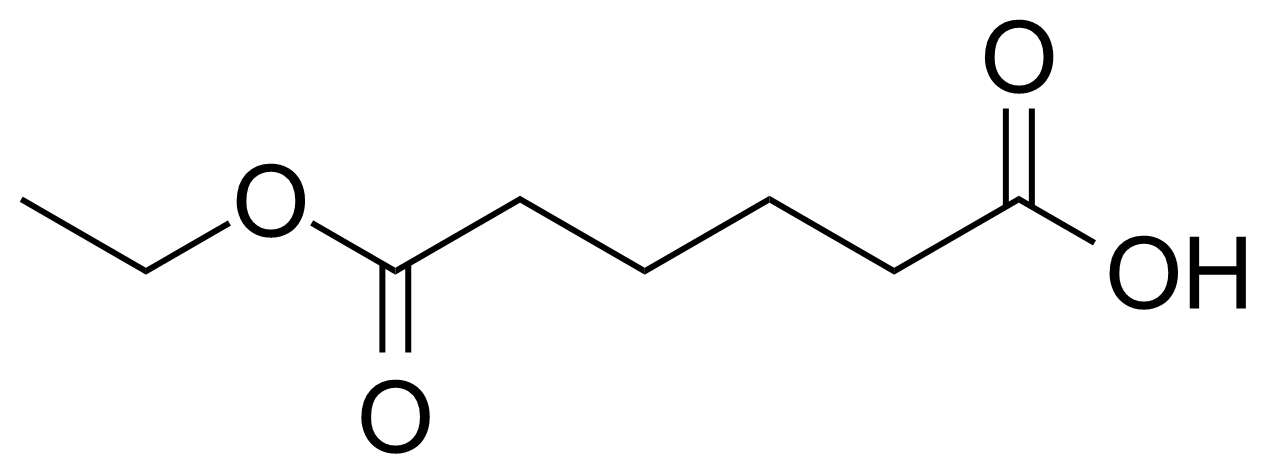 | [626-86-8] | GEO-00054 |
| New | 2-Aminoethyl methacrylate hydrochloride | 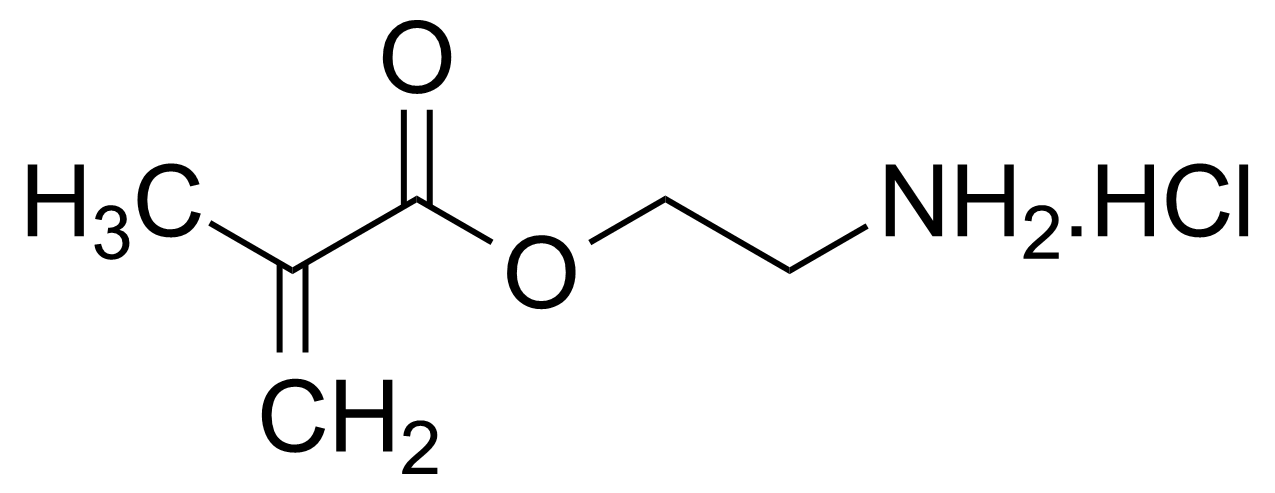 | [2420-94-2] | GEO-00132 |