| 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-beta-D-glucopyranosyl azide | 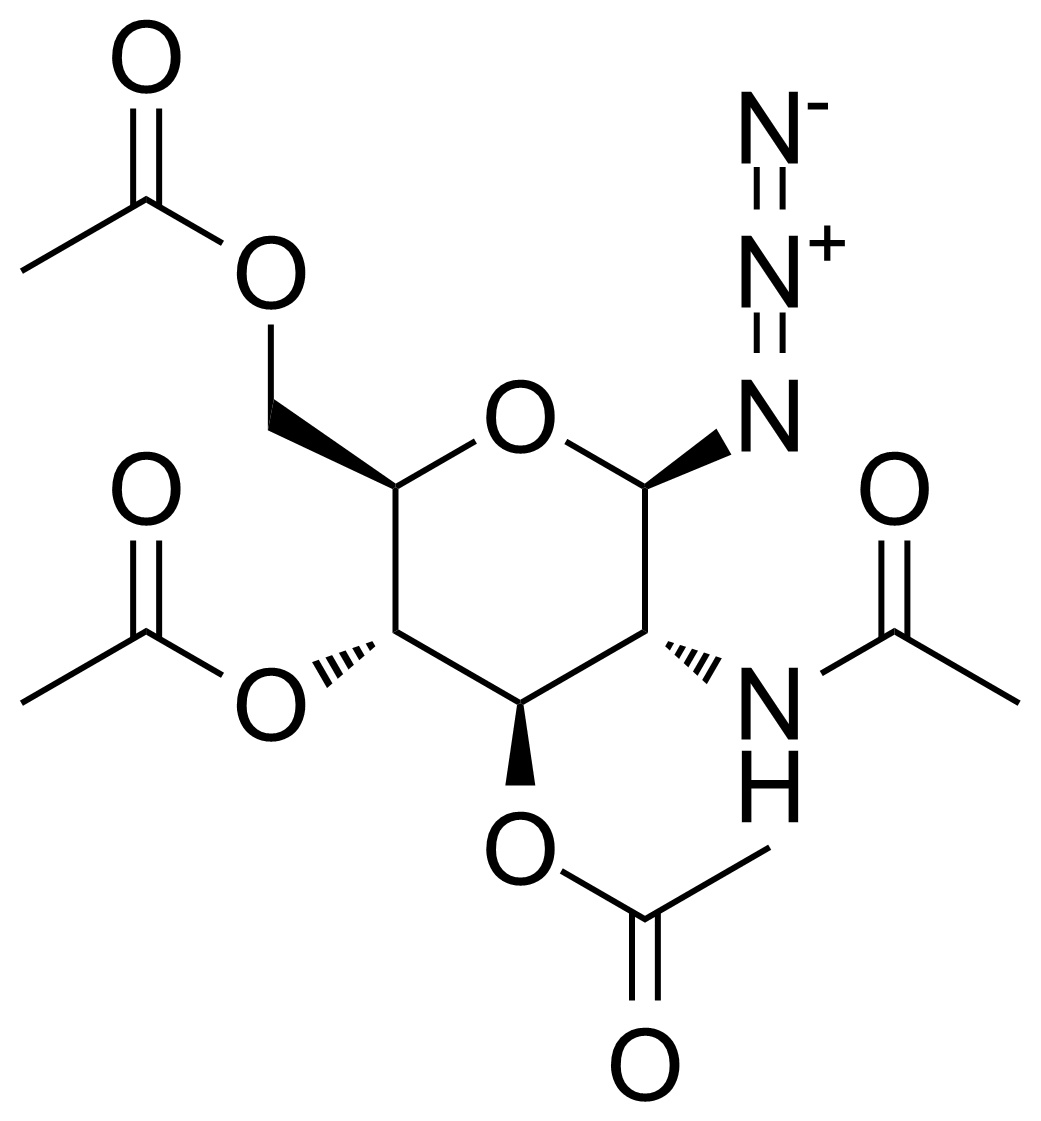 | [6205-69-2] | GEO-03200 |
| 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-alpha-D-glucopyranosyl chloride | 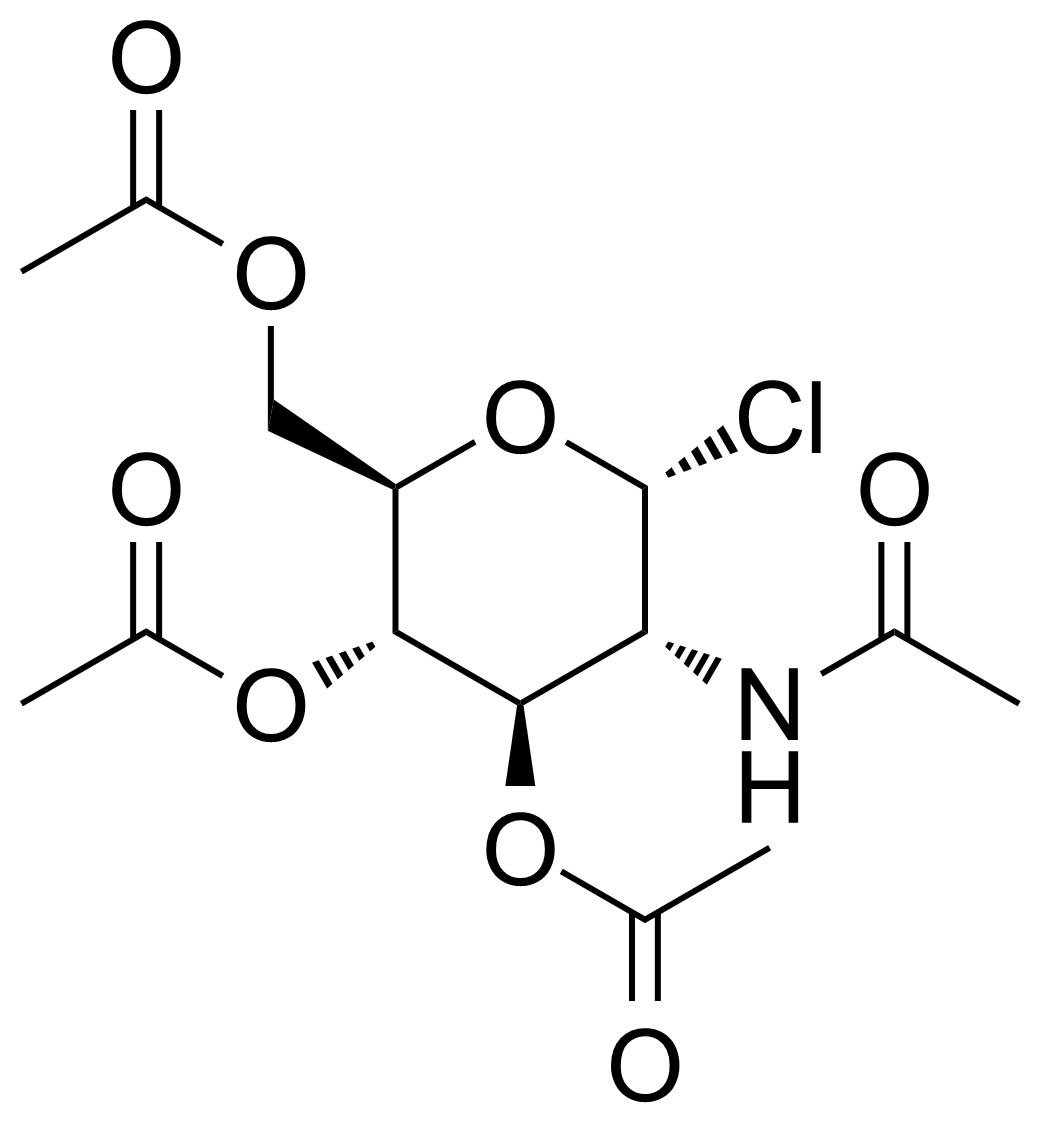 | [3068-34-6] | GEO-03374 |
| 2-Acetamido-1,3,4,6-tetra-O-acetyl-2-deoxy-beta-D-glucopyranose | 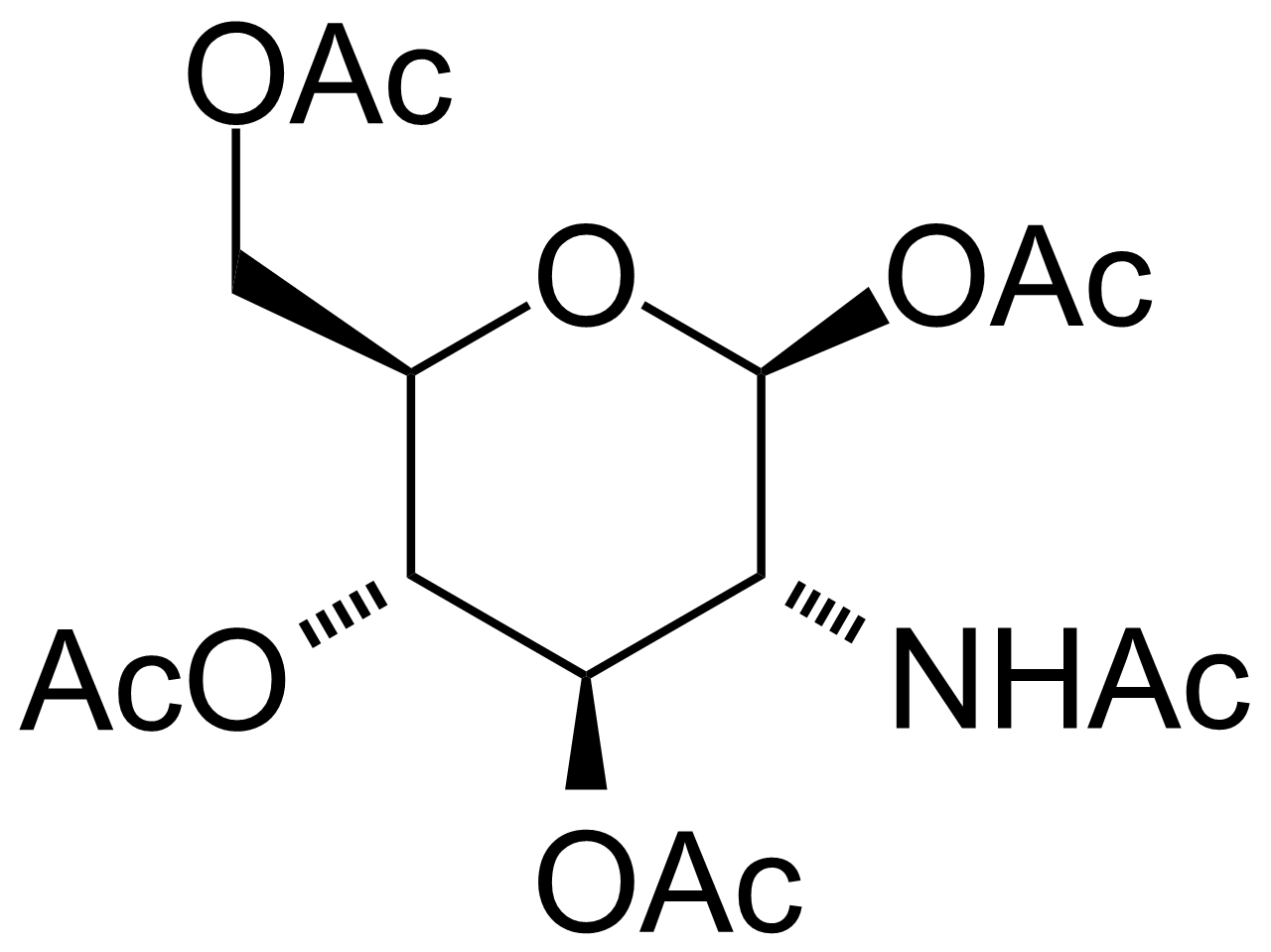 | [7772-79-4] | GEO-02610 |
| Acetochloro-beta-D-glucose | 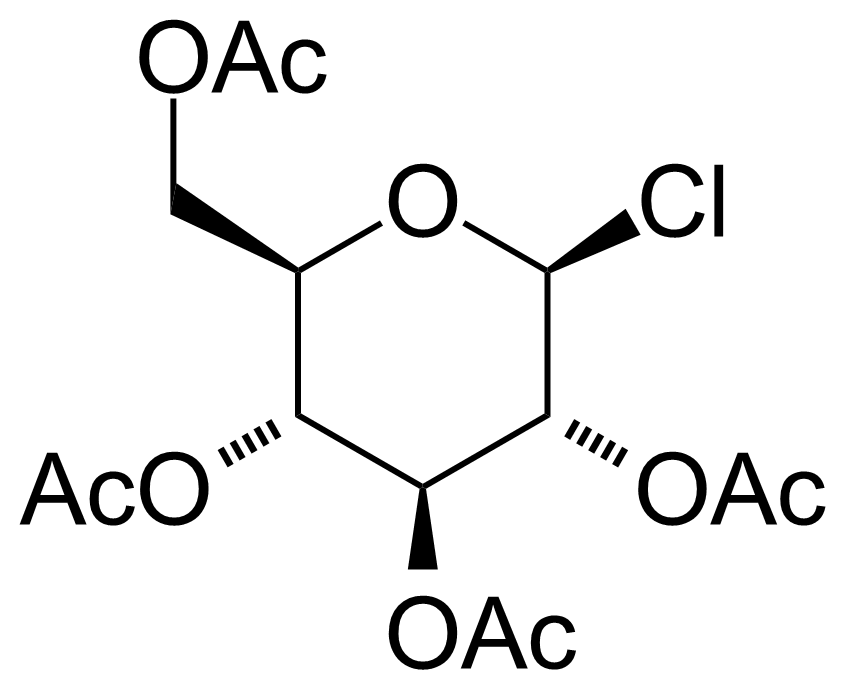 | [4451-36-9] | GEO-00008 |
| New | 5-Acetoxymethyl-2-furaldehyde | 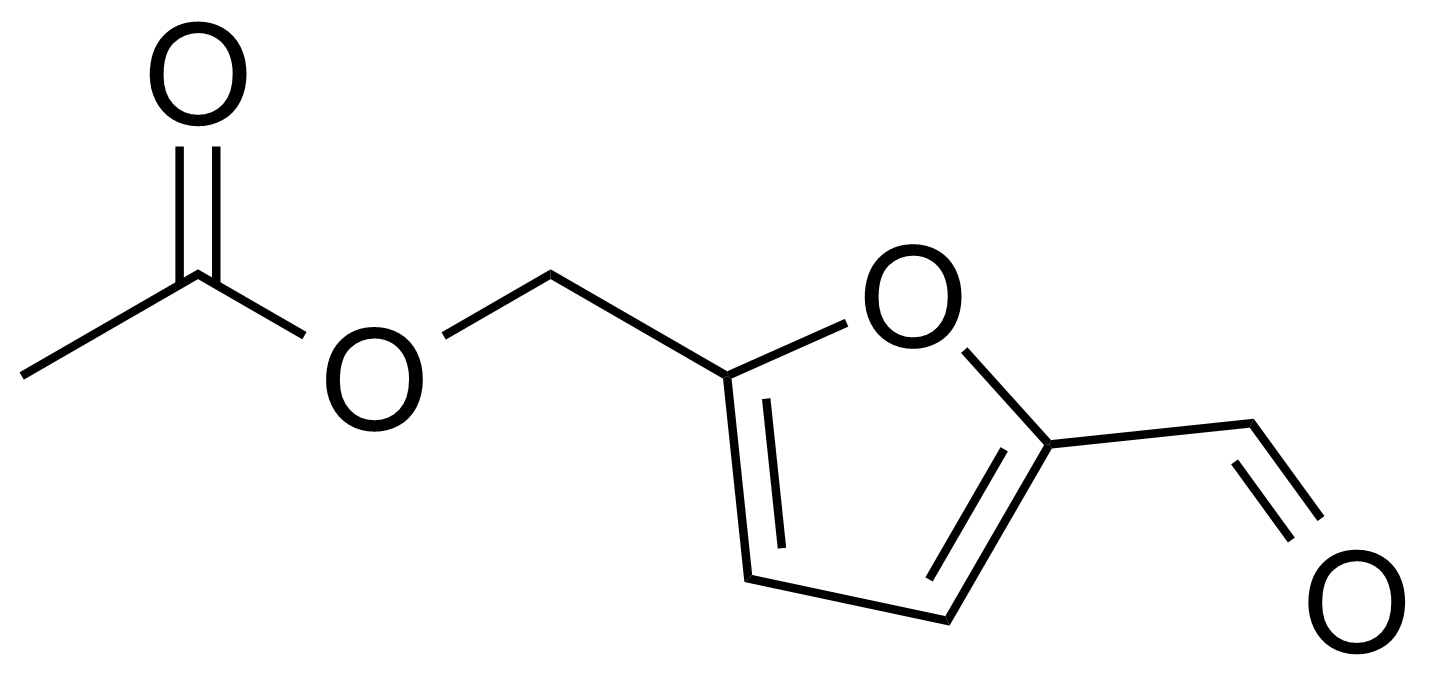 | [10551-58-3] | GEO-00013 |
| 2-Acetyl-4-methyl-4-pentenoic acid methyl ester | 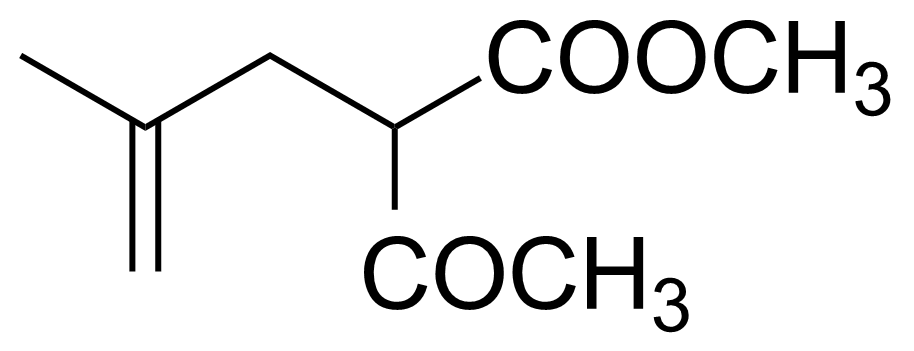 | [20962-71-4] | GEO-03240 |
| 5-(Acetyloxy)-2(5H)-furanone | 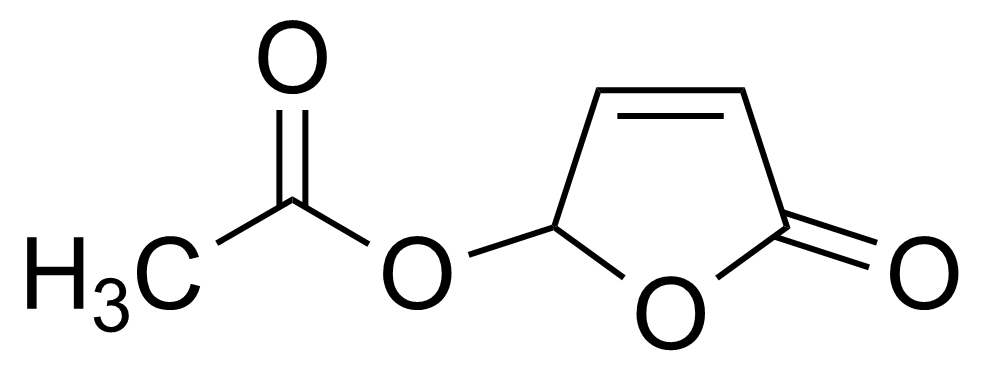 | [122952-20-9] | GEO-00007 |
| 1-Adamantyl methacrylate | 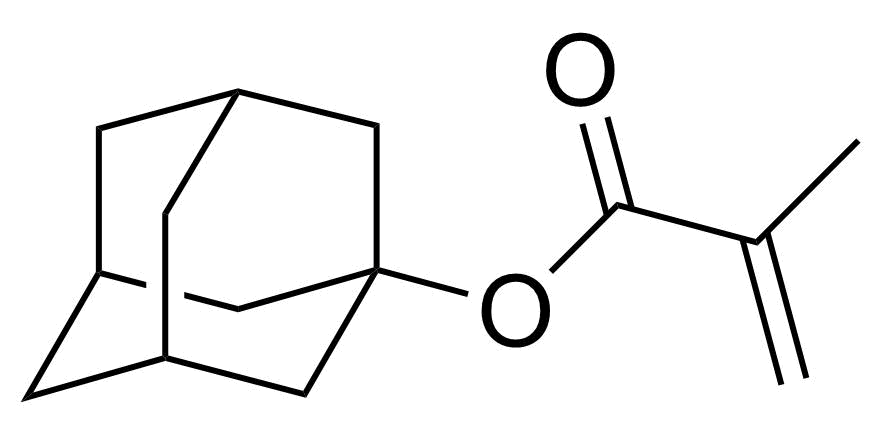 | [16887-36-8] | GEO-04510 |
| Adipic acid monoethyl ester | 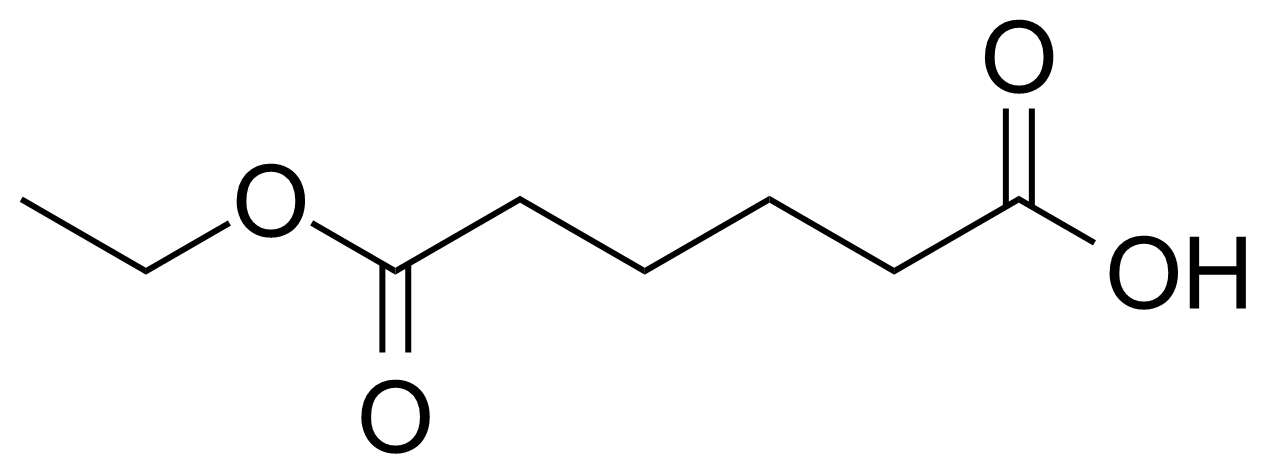 | [626-86-8] | GEO-00054 |
| 2-Aminoethyl methacrylate hydrochloride | 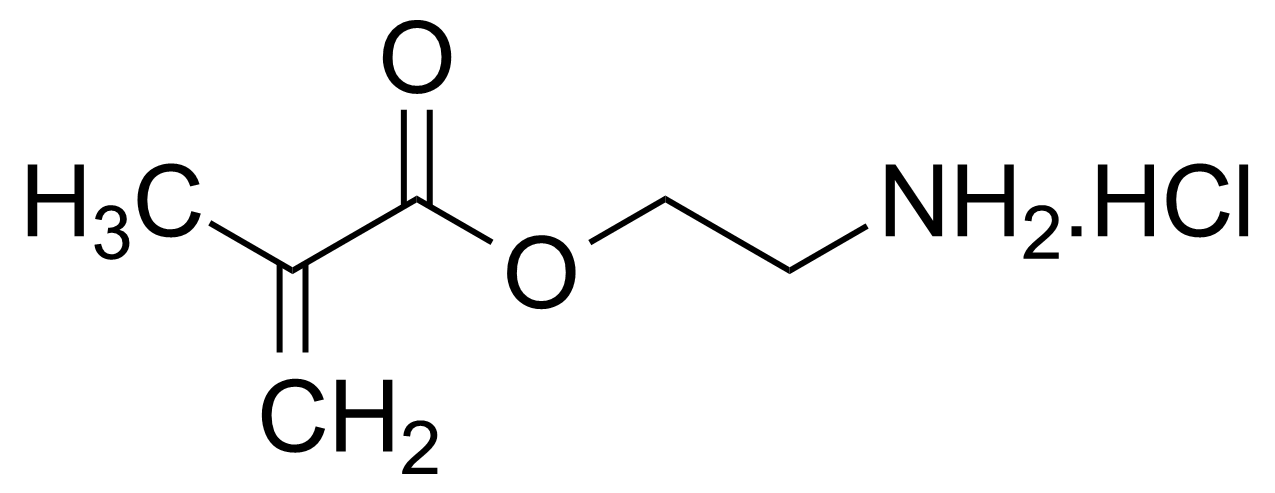 | [2420-94-2] | GEO-00132 |
| 7-Amino-4-methylcoumarin | 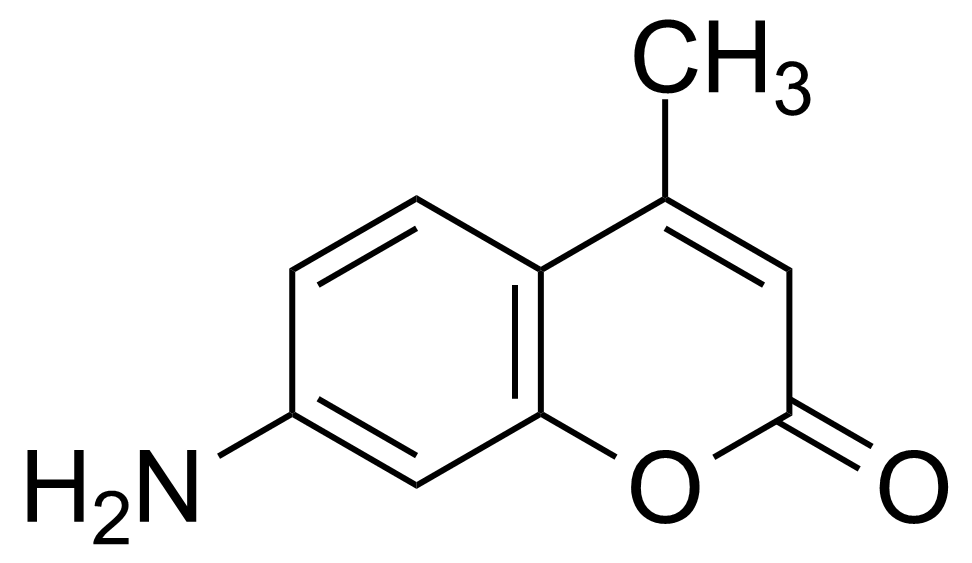 | [26093-31-2] | GEO-02697 |
| 6-Amino-2-naphthoic acid methyl ester | 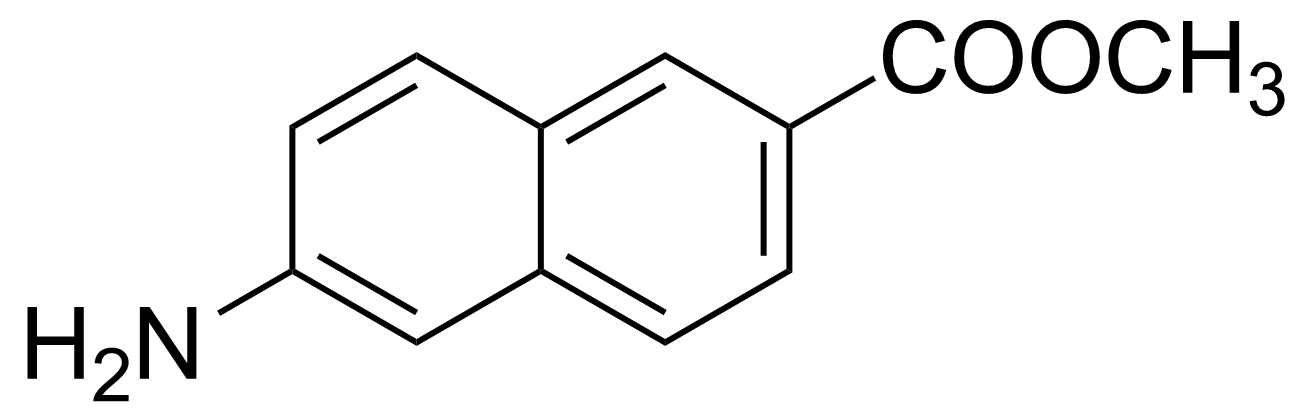 | [5159-59-1] | GEO-03262 |
| New | 1,6-Anhydro-beta-D-glucose-2,3,4-tri-O-acetate | 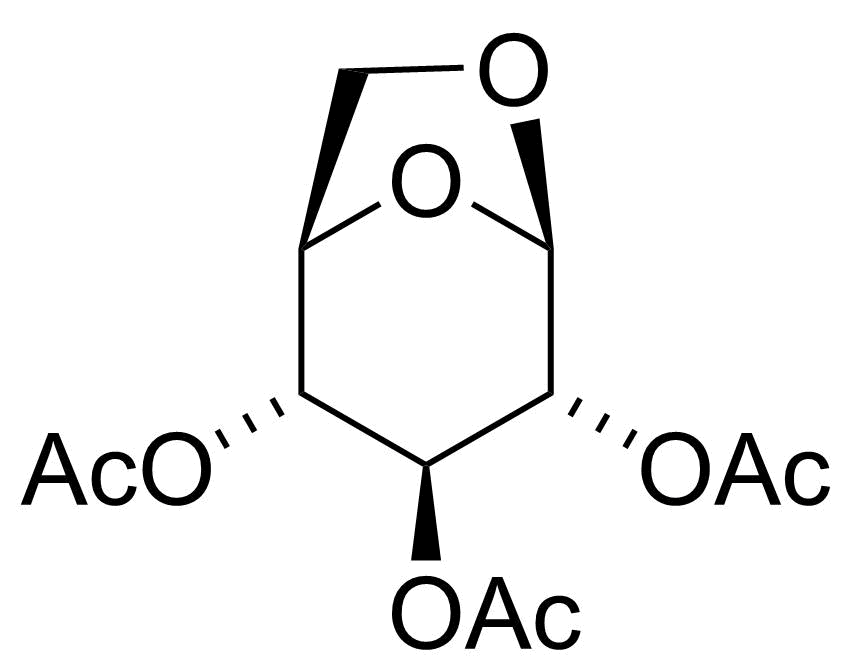 | [13242-55-2] | GEO-04689 |
| D-Arabinono-1,4-lactone | 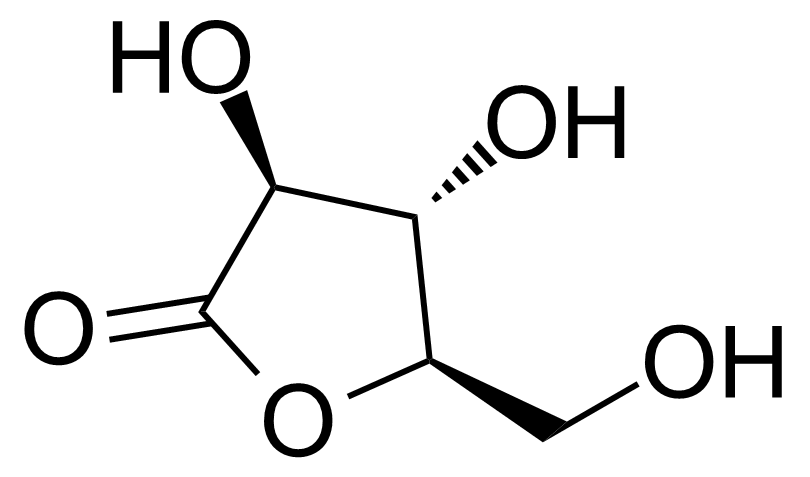 | [2782-09-4] | GEO-04321 |
| Benzo[b]thiophene-2-carboxylic acid methyl ester | ![Structure of Benzo[b]thiophene-2-carboxylic acid methyl ester](https://georganics.sk/wp-content/uploads/2021/05/GEO-00287_Benzobthiophene-2-carboxylic_acid_methyl_ester.png) | [22913-24-2] | GEO-00287 |
| 2,3,5-Tri-o-benzyl-d-arabino-1,4-lactone | 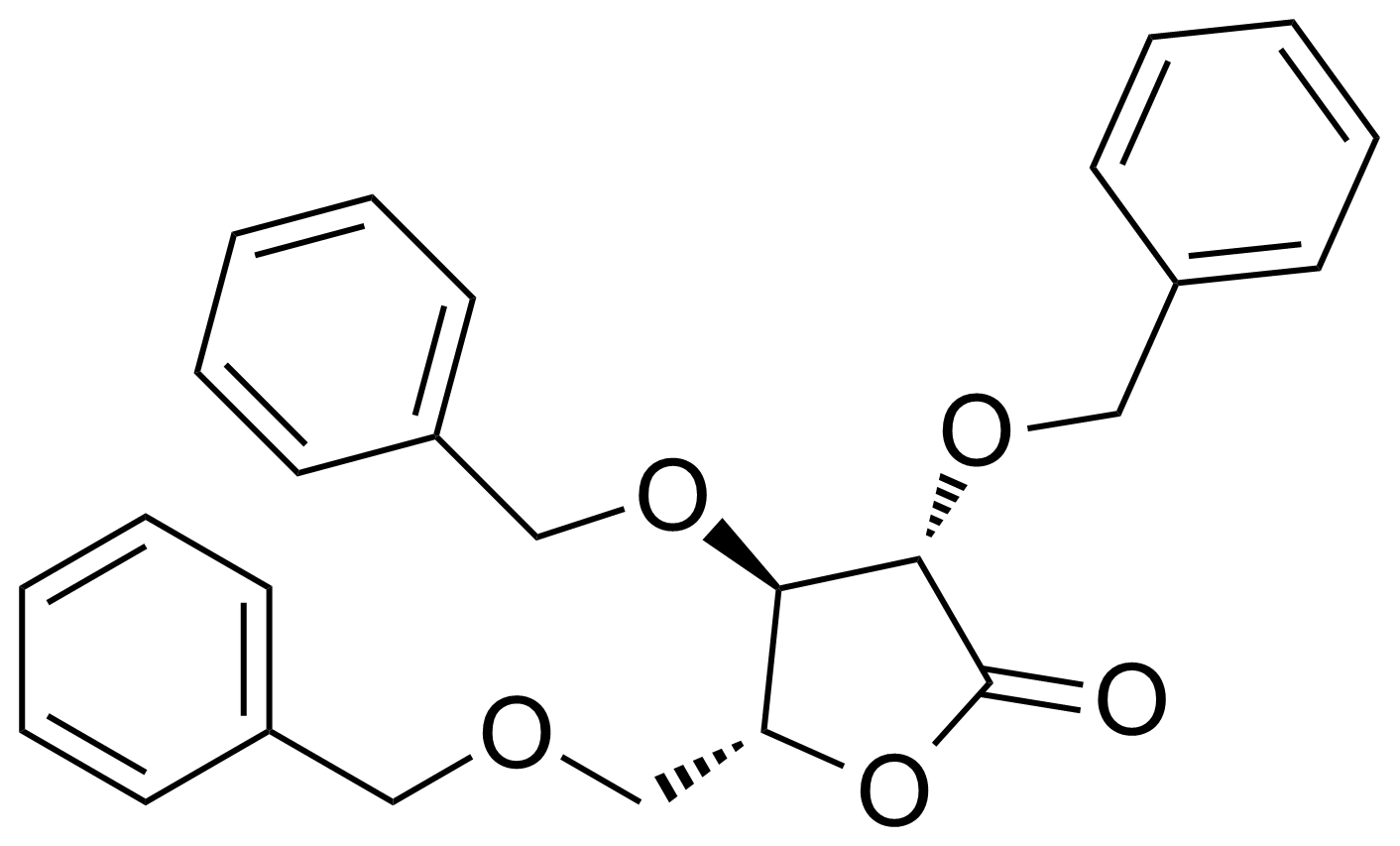 | [14233-64-8] | GEO-04322 |
| 2-Benzyloxycarbonyl-3-ethyl-4-methylpyrrole | 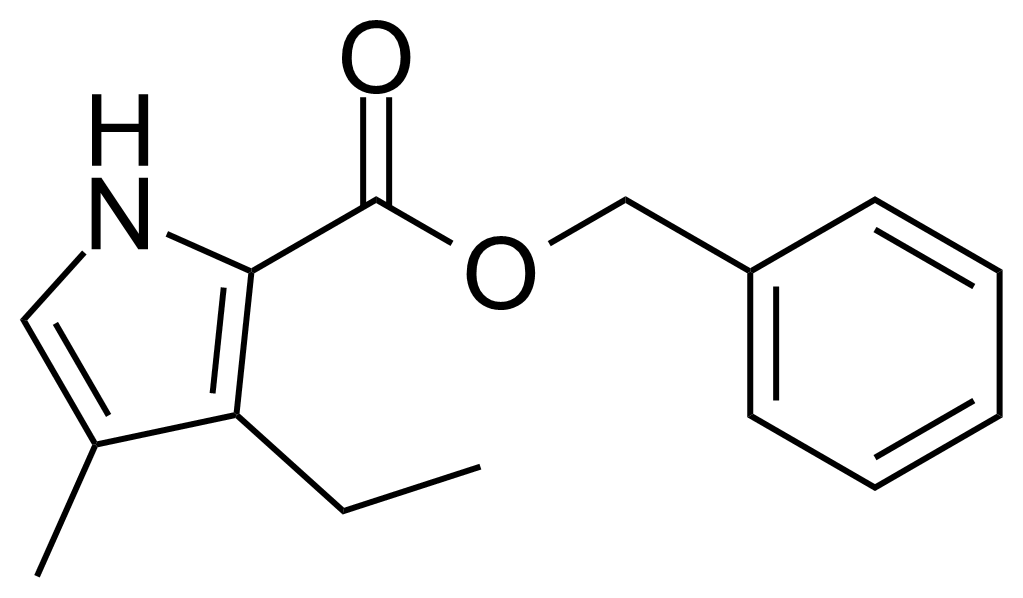 | [5866-56-8] | GEO-04133 |
| Benzyl piperidine-4-carboxylate | 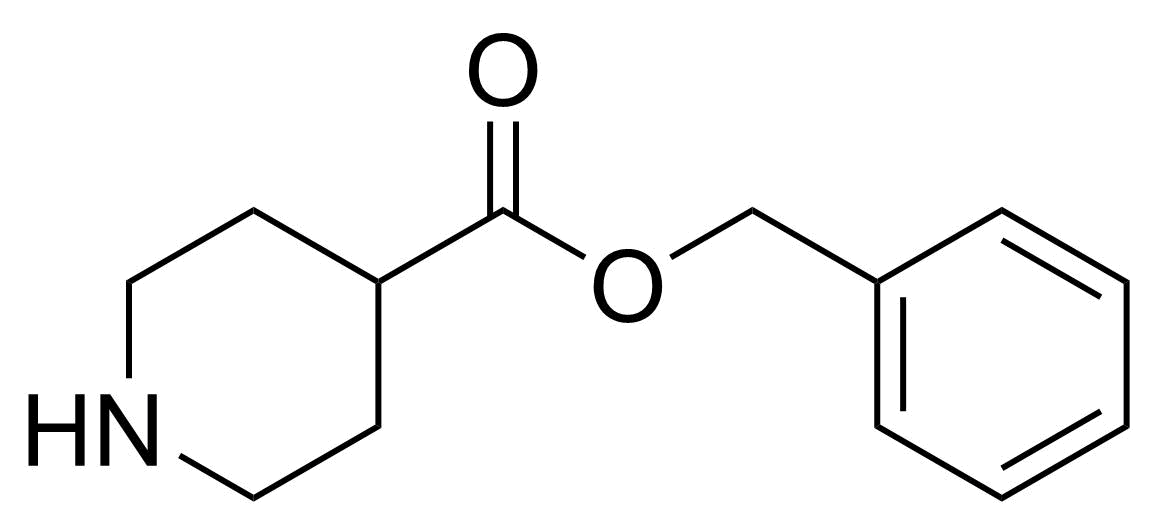 | [103824-89-1] | GEO-04512 |
| Bromomethyl acetate | 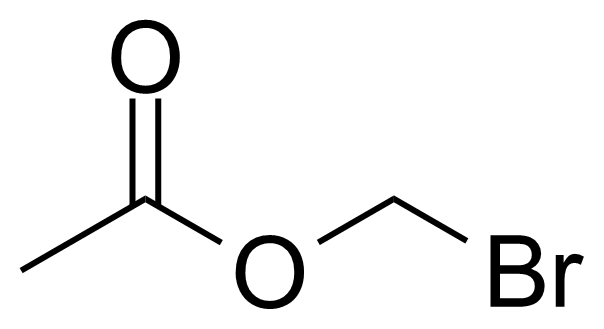 | [590-97-6] | GEO-02798 |
| n-Butyl isocyanatoacetate | 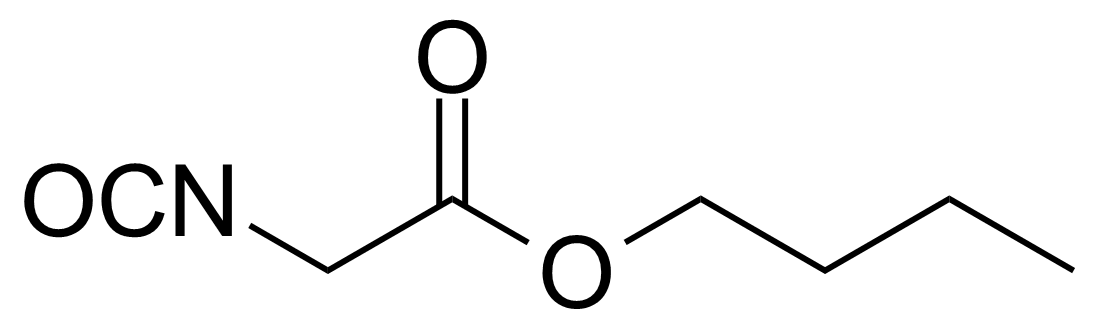 | [17046-22-9] | GEO-03693 |
| tert-Butyl methyl malonate | 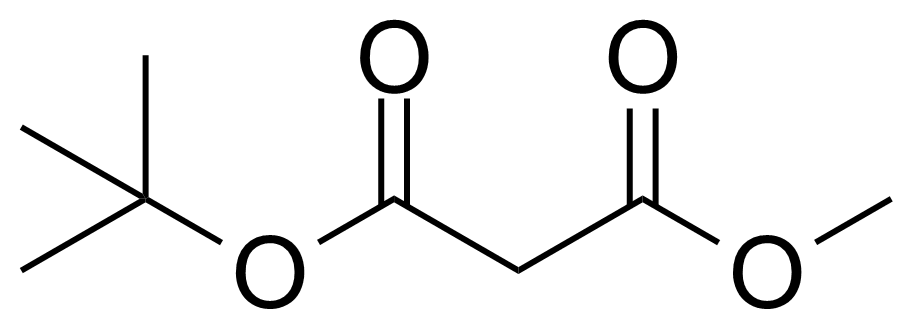 | [42726-73-8] | GEO-00623 |
| (+)-Camptothecin | 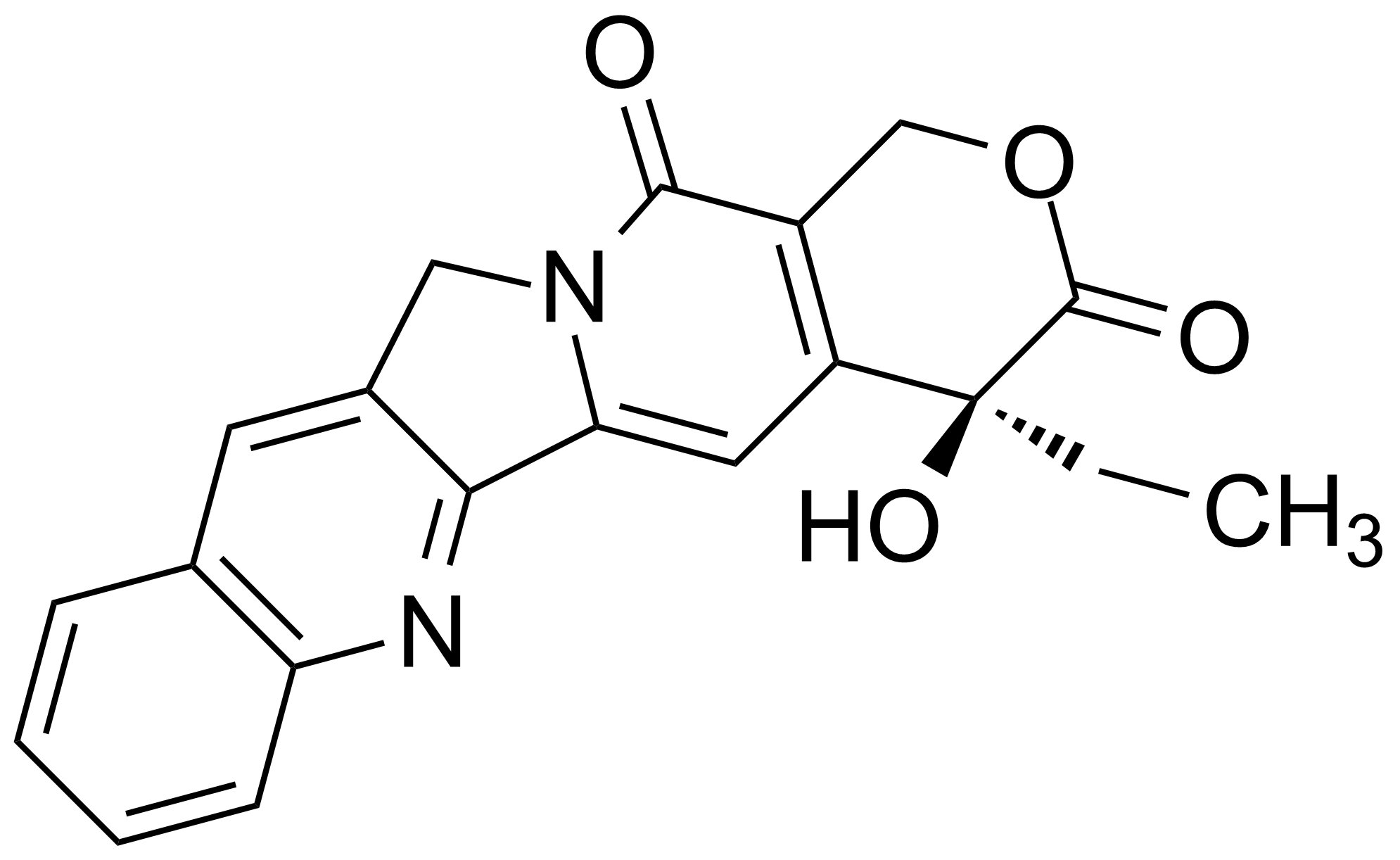 | [7689-03-4] | GEO-02663 |
| 3-Carbomethoxyphenyl isocyanate | 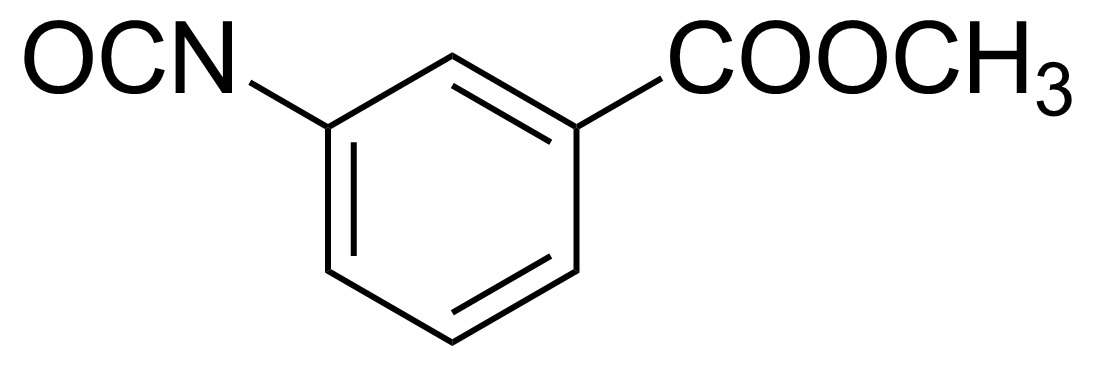 | [41221-47-0] | GEO-00638 |
| 3,3′-Carbonylbis(7-N,N-diethylaminocoumarin) | 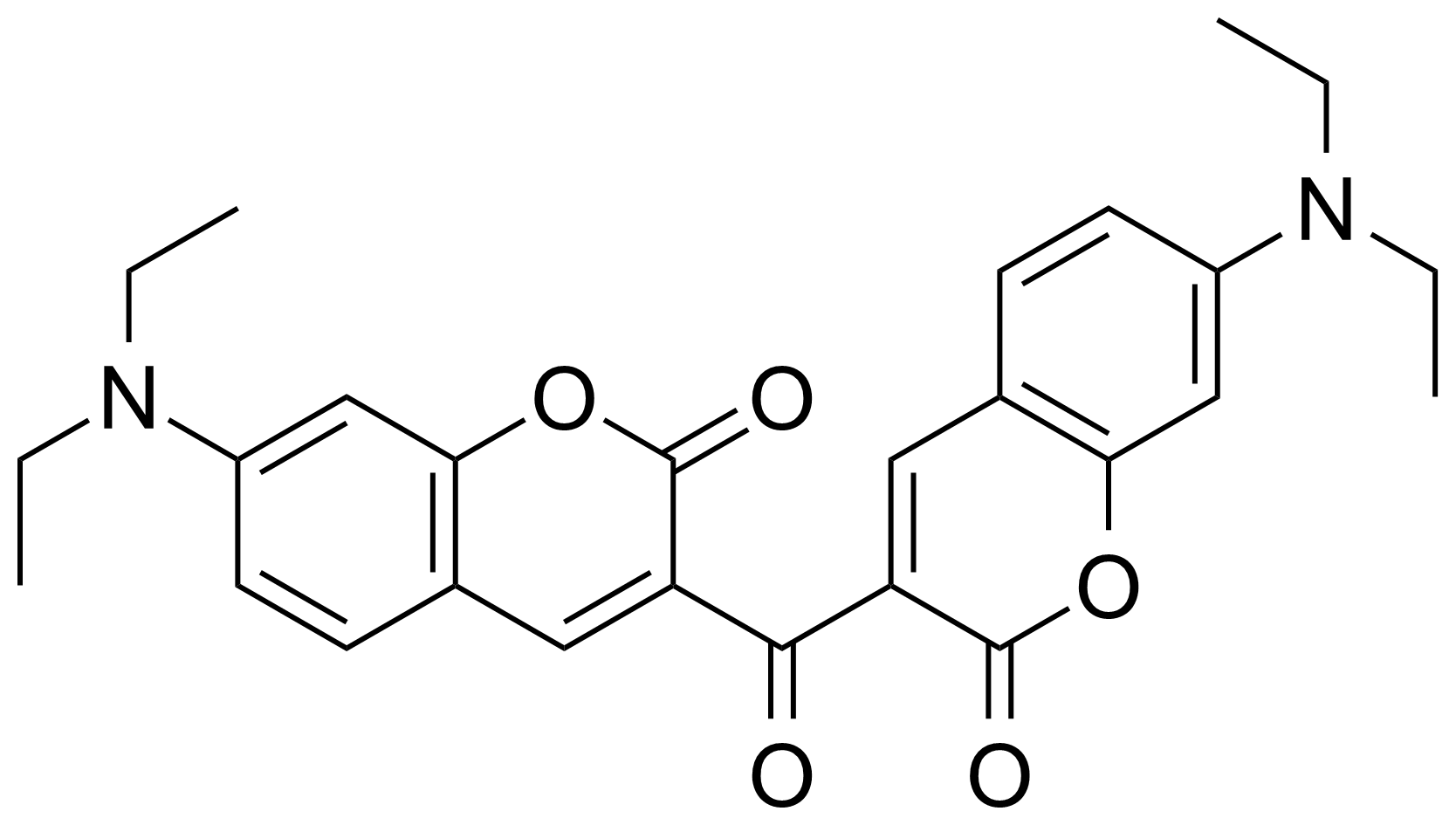 | [63226-13-1] | GEO-04431 |
| 2-Chloro-3-hydroxypropionic acid methyl ester | 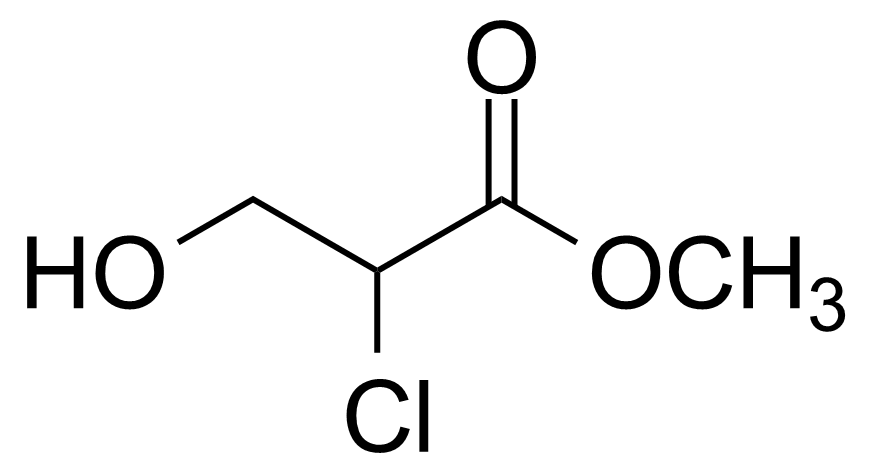 | [98070-39-4] | GEO-00689 |
| 4-Chloropicolinic acid methyl ester | 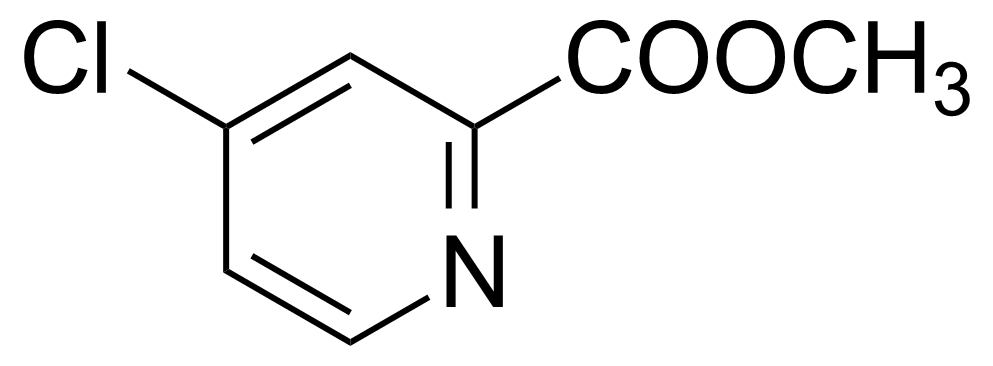 | [24484-93-3] | GEO-00791 |
| 3-Chloro-5-trifluoromethylpyridine-2-carboxylic acid ethyl ester |  | [128073-16-5] | GEO-03228 |
| 3-Cyano-1H-pyrazole-4-carboxylic acid methylester | 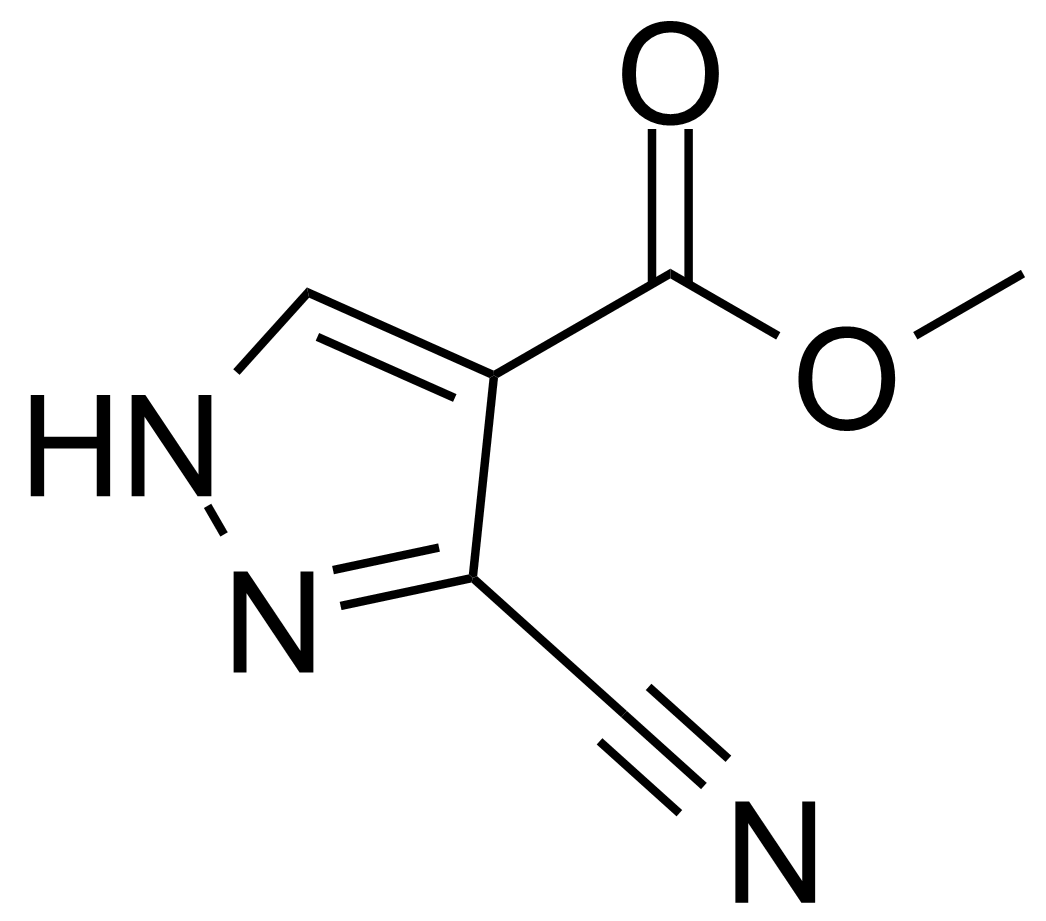 | [33090-69-6] | GEO-00850 |
| 4-Cyclopentylamino-2-methanethiopyrimidine-5-carboxylic acid ethyl ester | 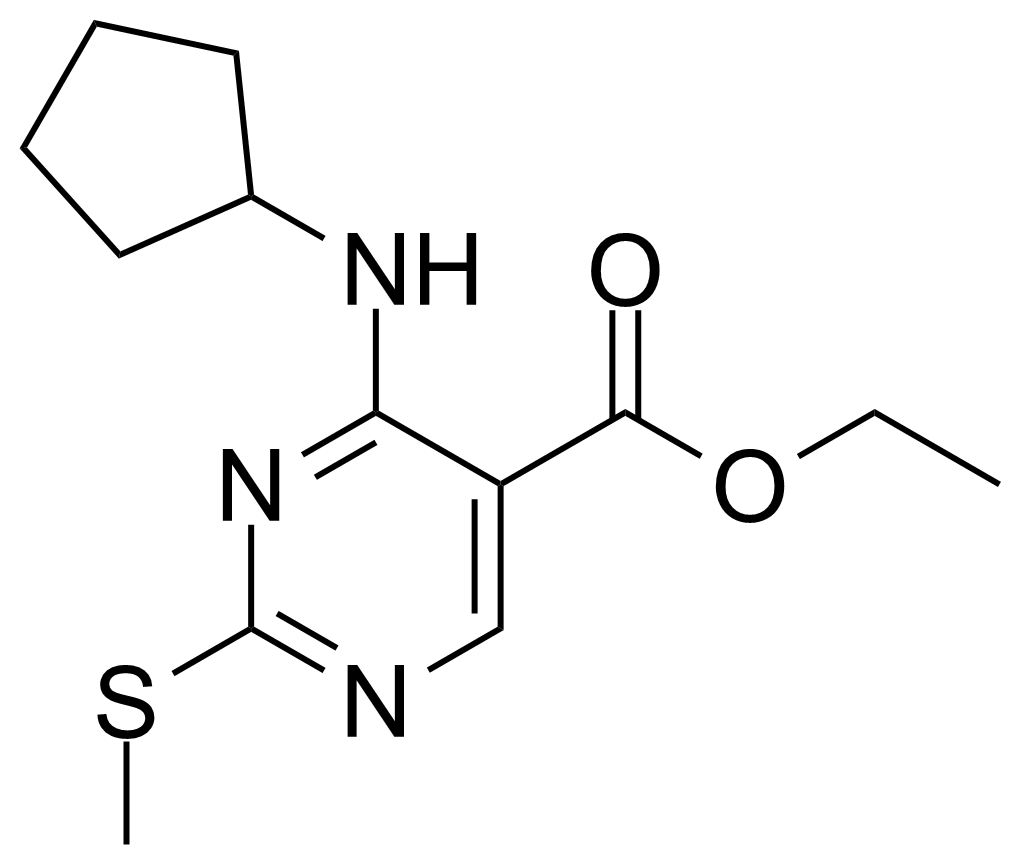 | [211245-62-4] | GEO-04295 |
| Diethyl acetamidomalonate | 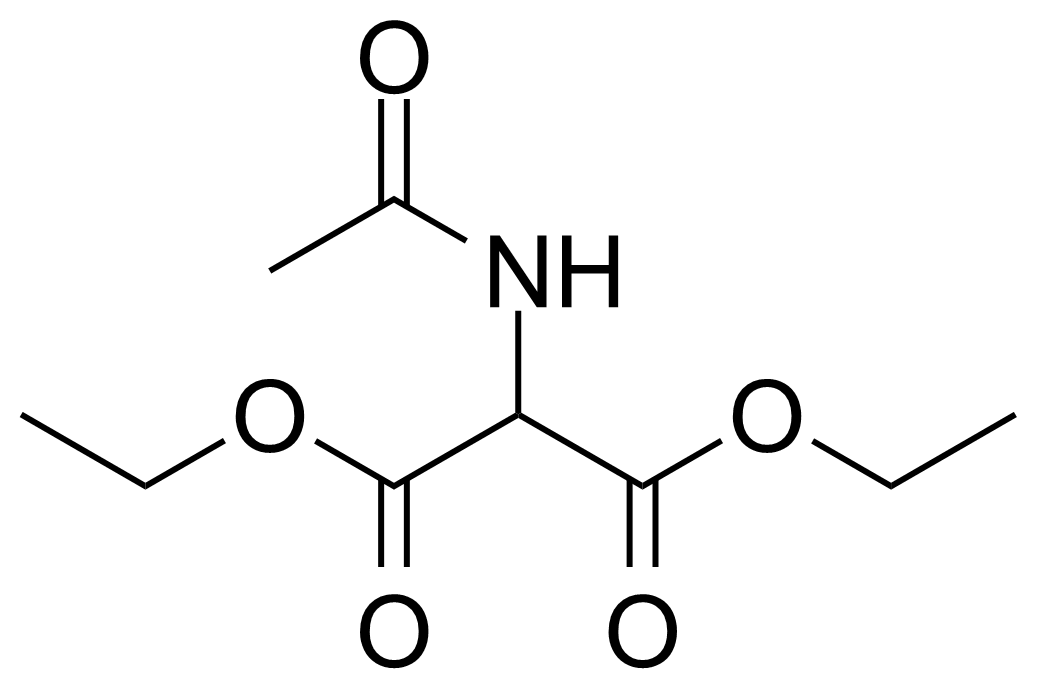 | [1068-90-2] | GEO-01032 |
| Diethyl 2-[4-(benzyloxy)benzylidene]malonate | ![Structure of Diethyl 2-[4-(benzyloxy)benzylidene]malonate](https://georganics.sk/wp-content/uploads/2021/06/GEO-01038_Diethyl_2-4-benzyloxybenzylidenemalonate.png) | [53361-40-3] | GEO-01038 |
| Diethyl 3′-bromo-2,6-dimethyl-1,4-dihydro-[4,4′-bipyridine]-3,5-dicarboxylate | 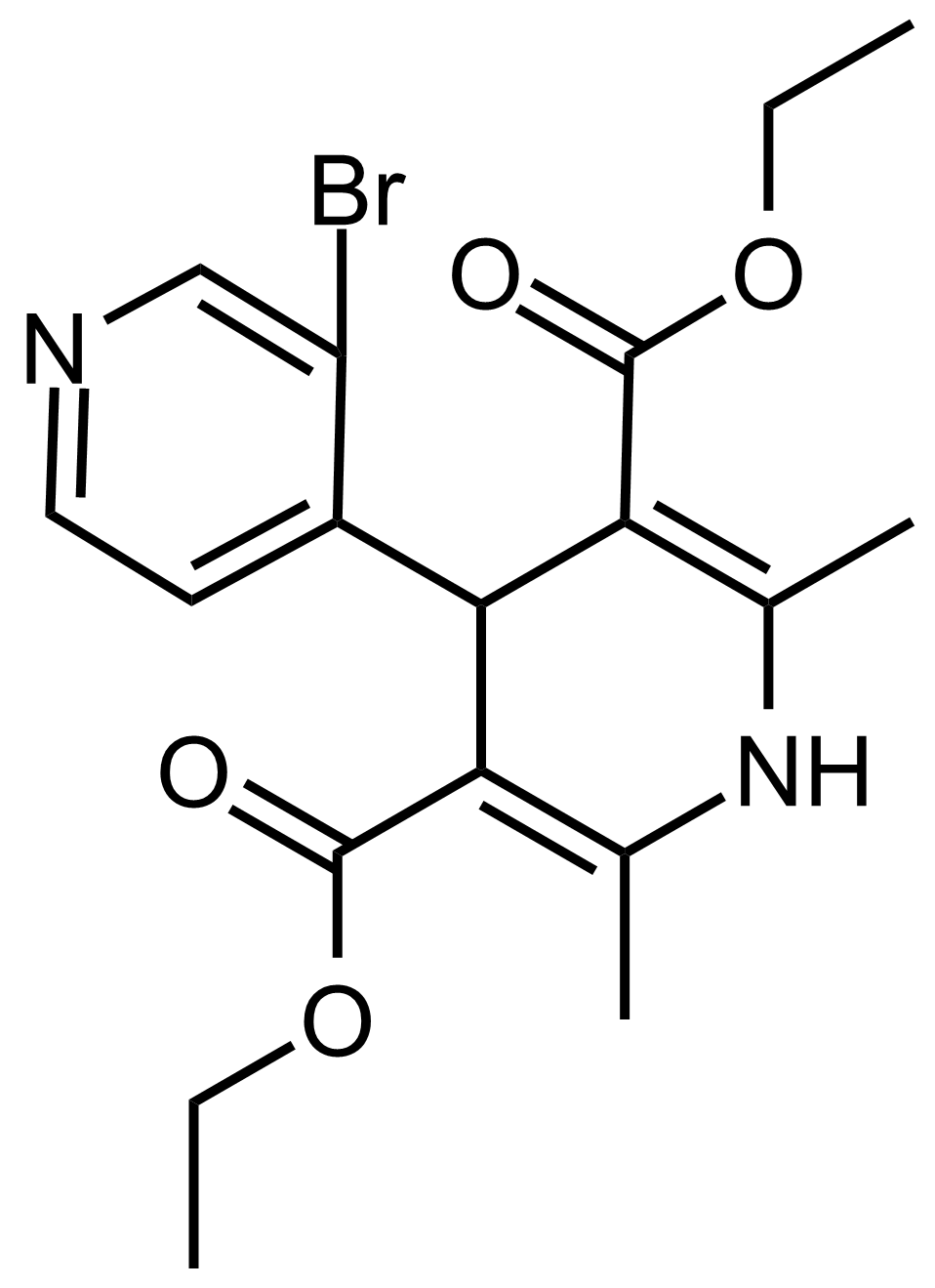 | N/A | GEO-03567 |
| Diethyl 2,6-dichloro-2′,6′-dimethyl-1′,4′-dihydro-[3,4′-bipyridine]-3′,5′-dicarboxylate | 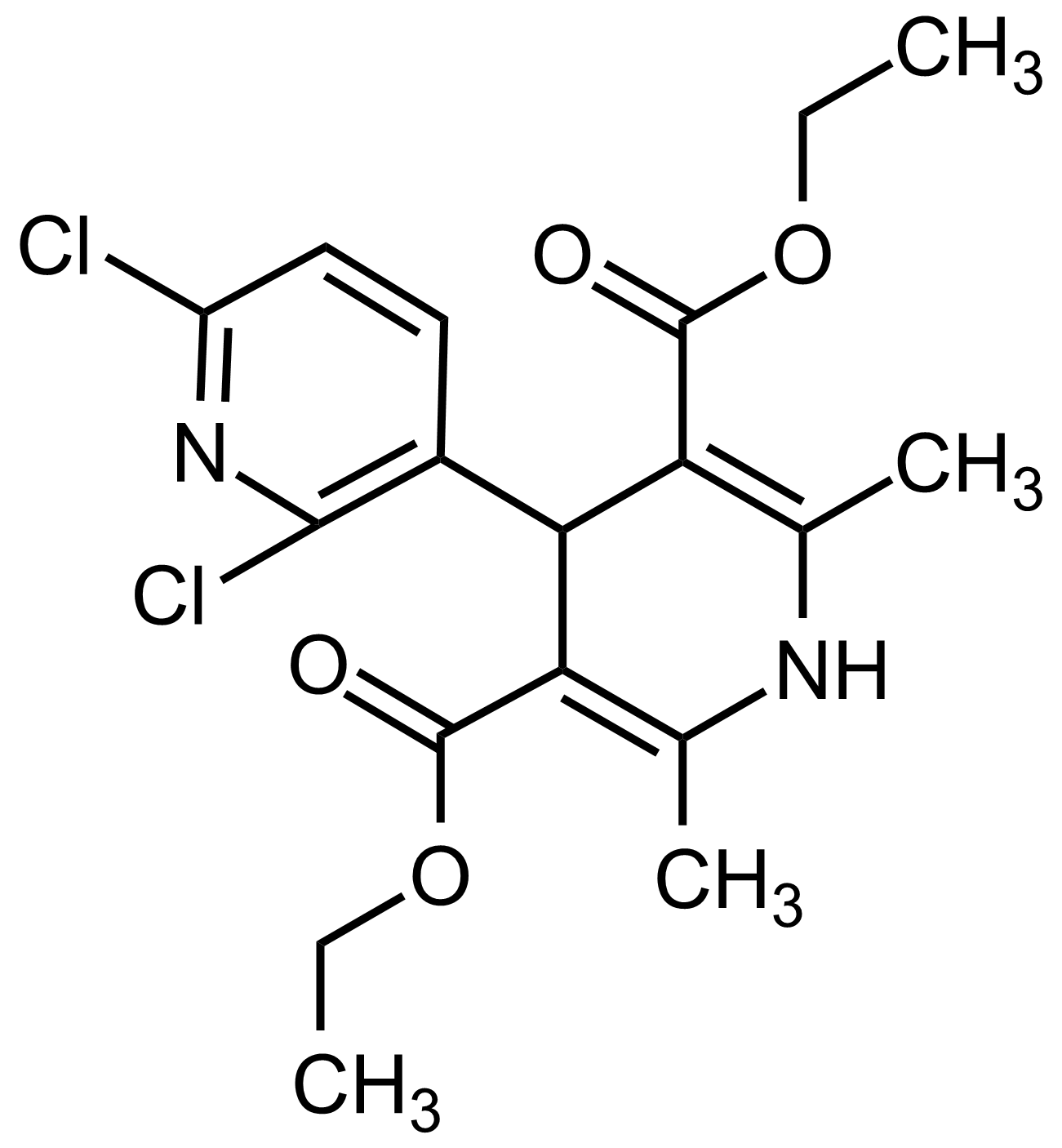 | N/A | GEO-03552 |
| Diethyl 2,6-dimethyl-4-(5-methylfuran-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 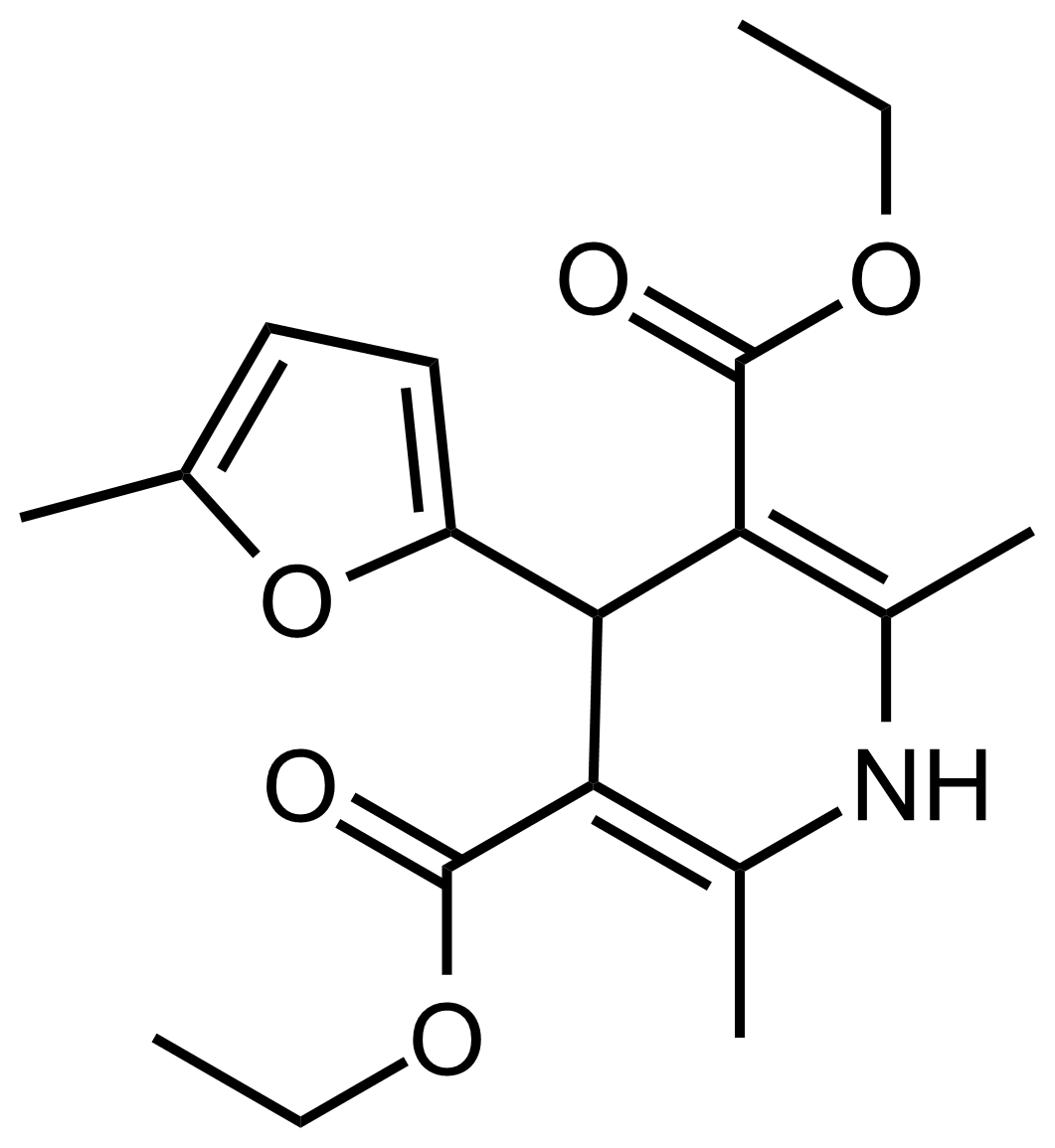 | N/A | GEO-03575 |
| Diethyl 2,6-dimethyl-4-(5-((5-methyl-1,3,4-thiadiazol-2-yl)thio)furan-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate |  | N/A | GEO-03540 |
| Diethyl 2,6-dimethyl-4-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 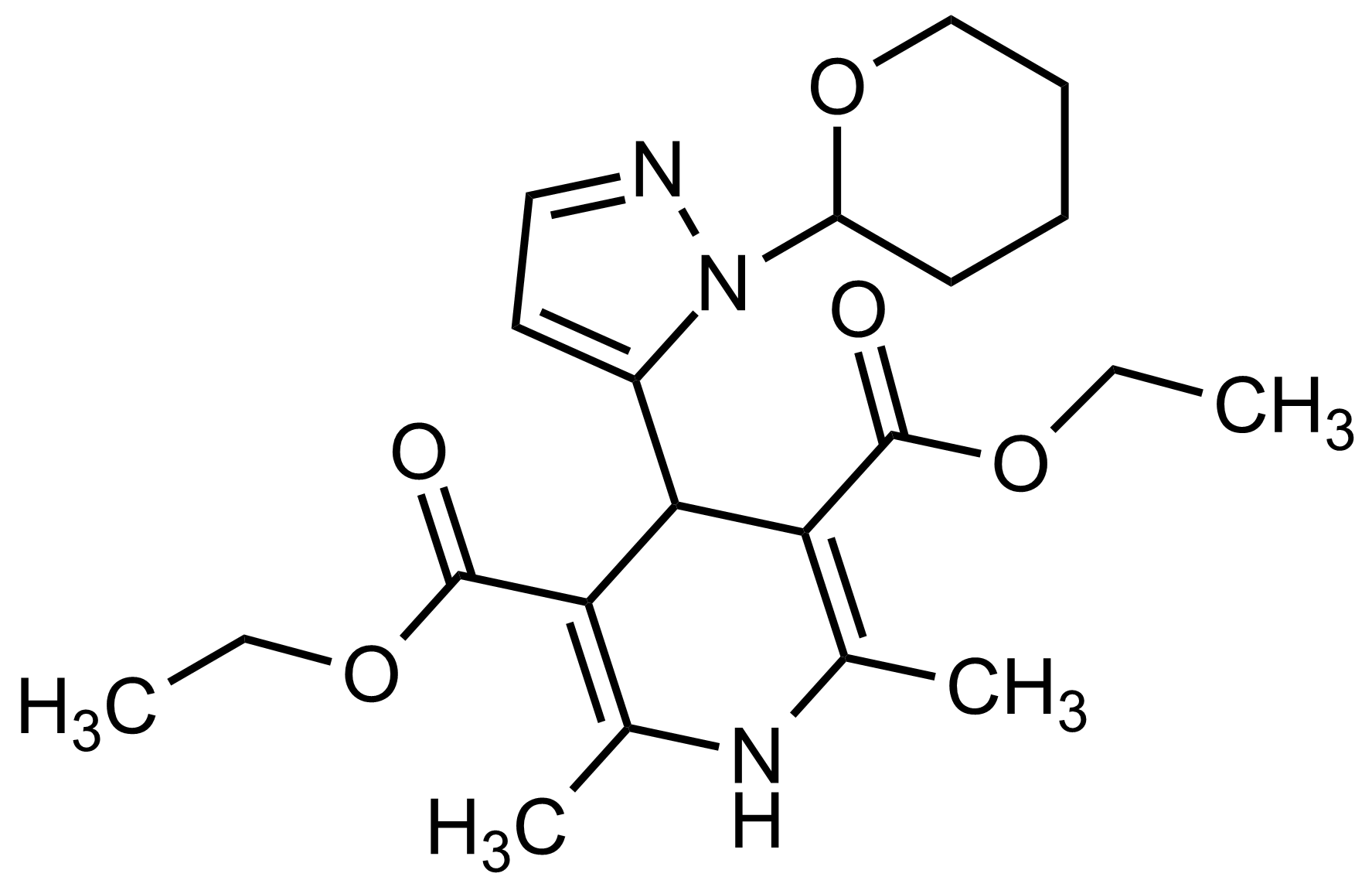 | N/A | GEO-03542 |
| Diethyl meso-2,5-dibromoadipate | 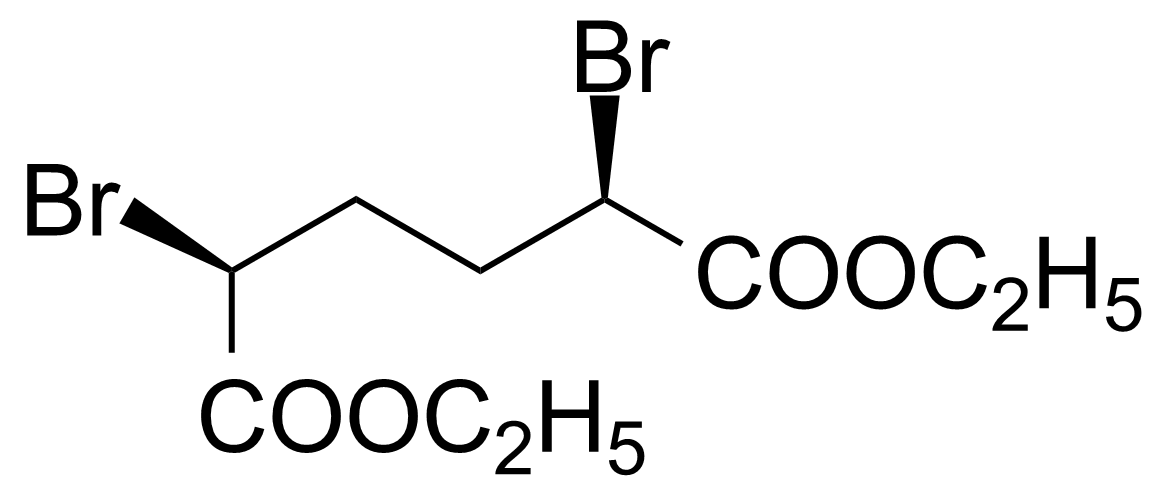 | [54221-37-3] | GEO-01041 |
| Diethyl 2′,3,6′-trimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate | 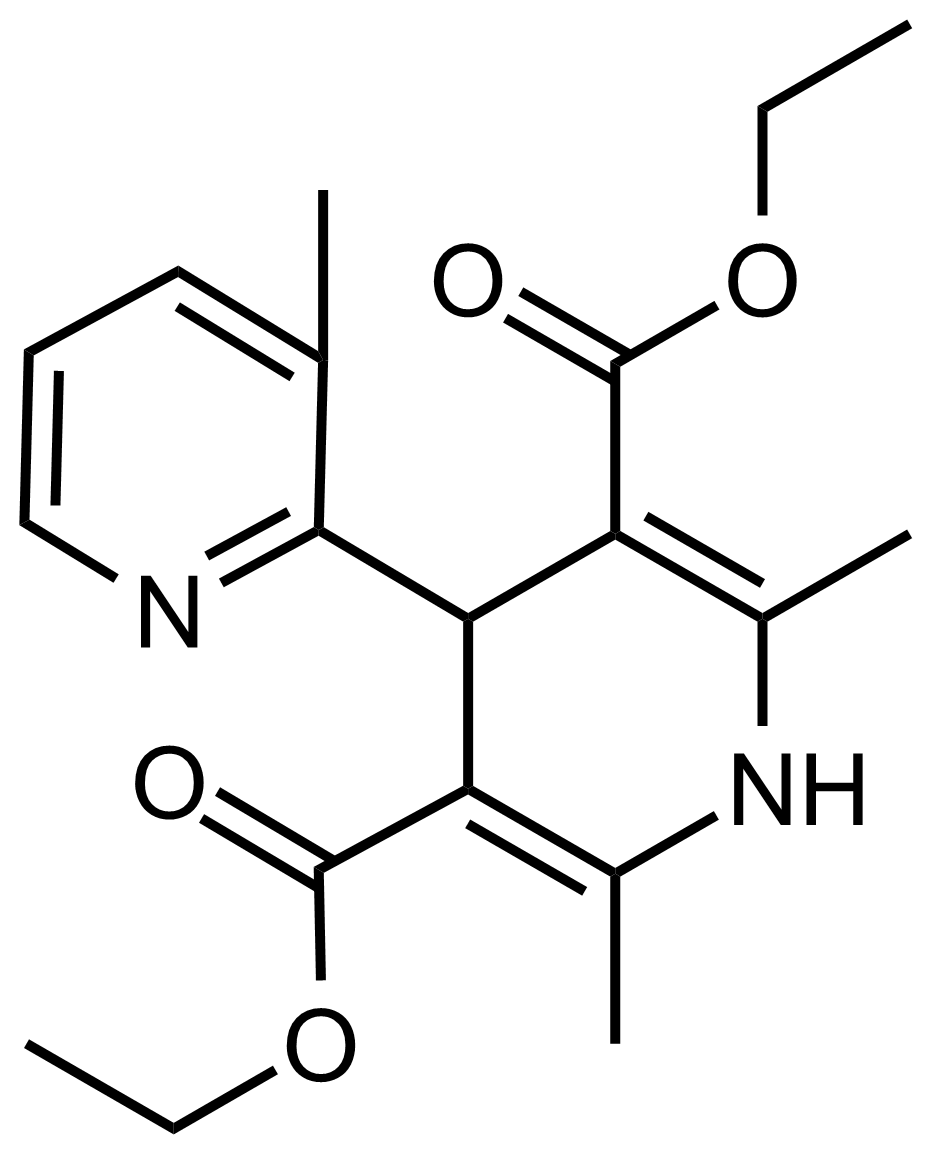 | N/A | GEO-03534 |
| Diethyl 2′,5,6′-trimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate | 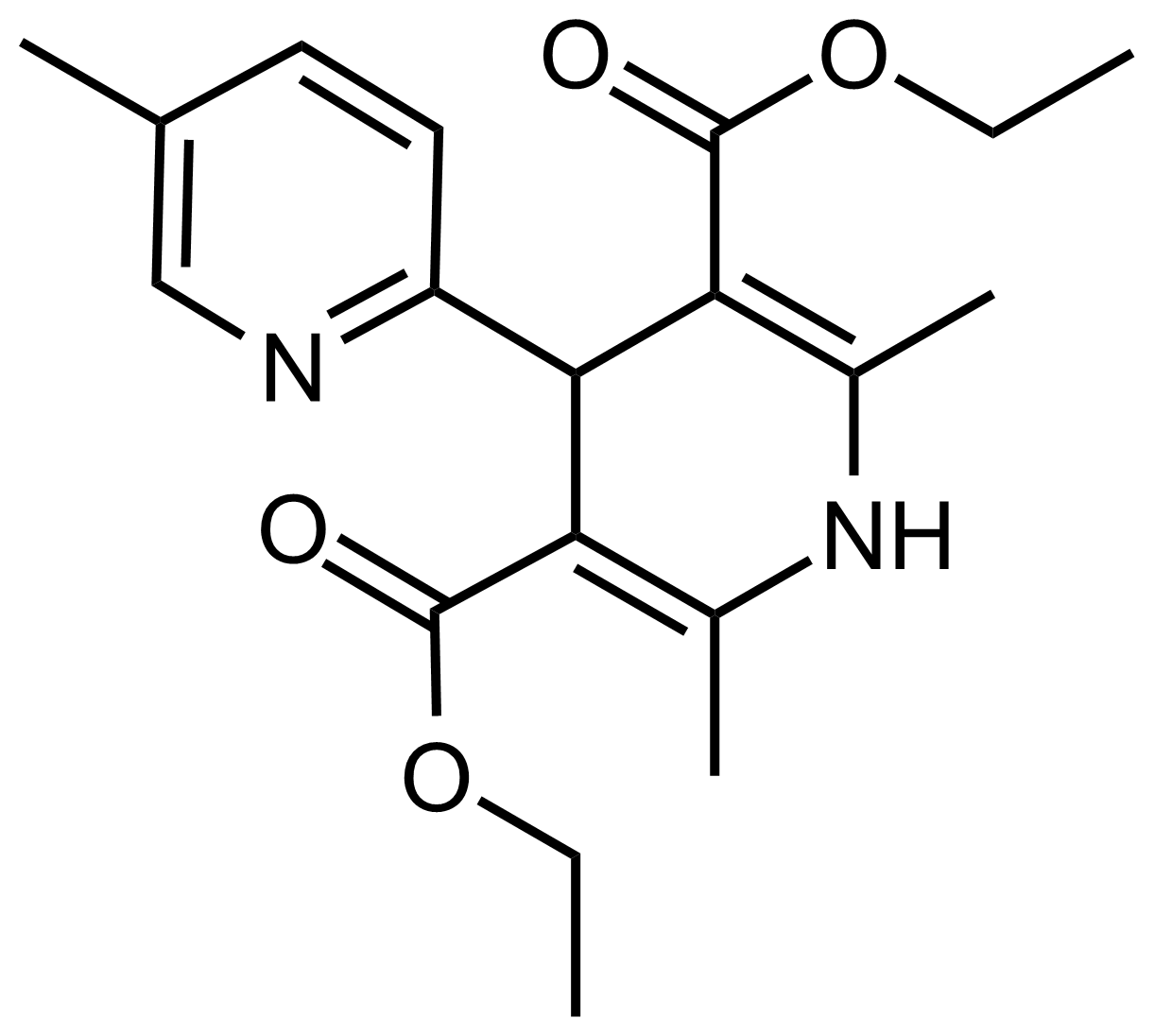 | N/A | GEO-03538 |
| (S)-4,5-Dihydro-4-hydroxy-2(3H)-furanone | 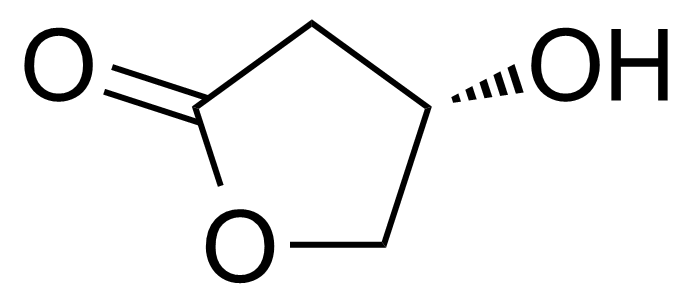 | [7331-52-4] | GEO-03952 |
| 3,4-Dihydroxy-5-methoxybenzoic acid methyl ester | 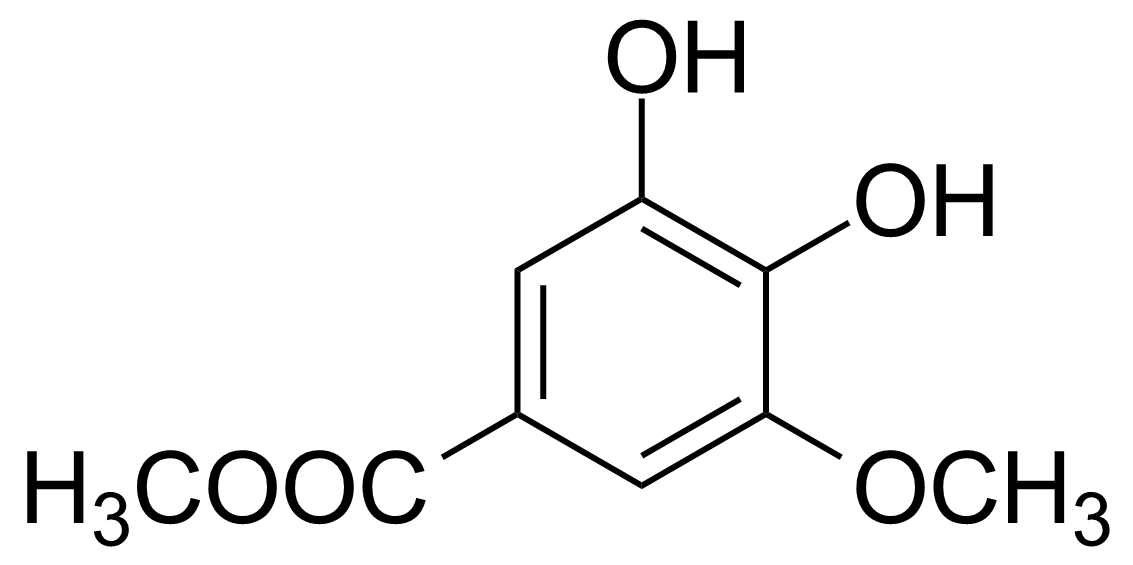 | [3934-86-9] | GEO-01104 |
| Dimethyl 1,3-adamantanedicarboxylate | 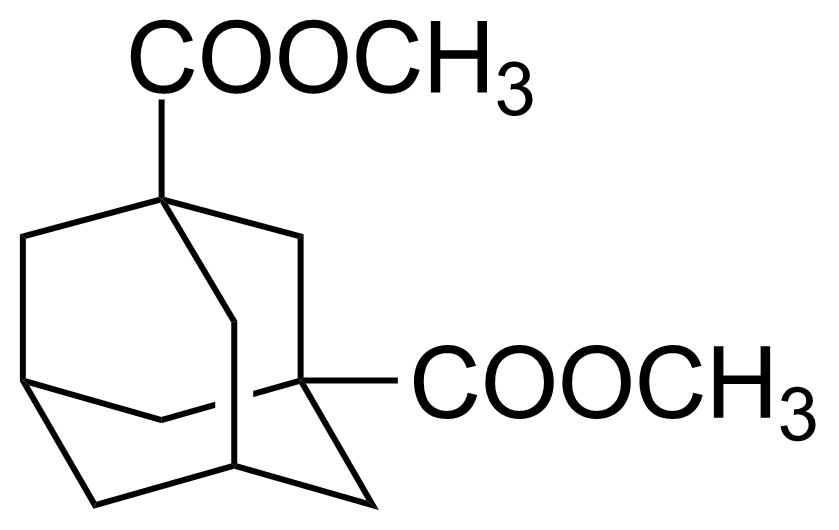 | [1459-95-6] | GEO-01144 |
| Dimethyl 3′-bromo-2,6-dimethyl-1,4-dihydro-[4,4′-bipyridine]-3,5-dicarboxylate | 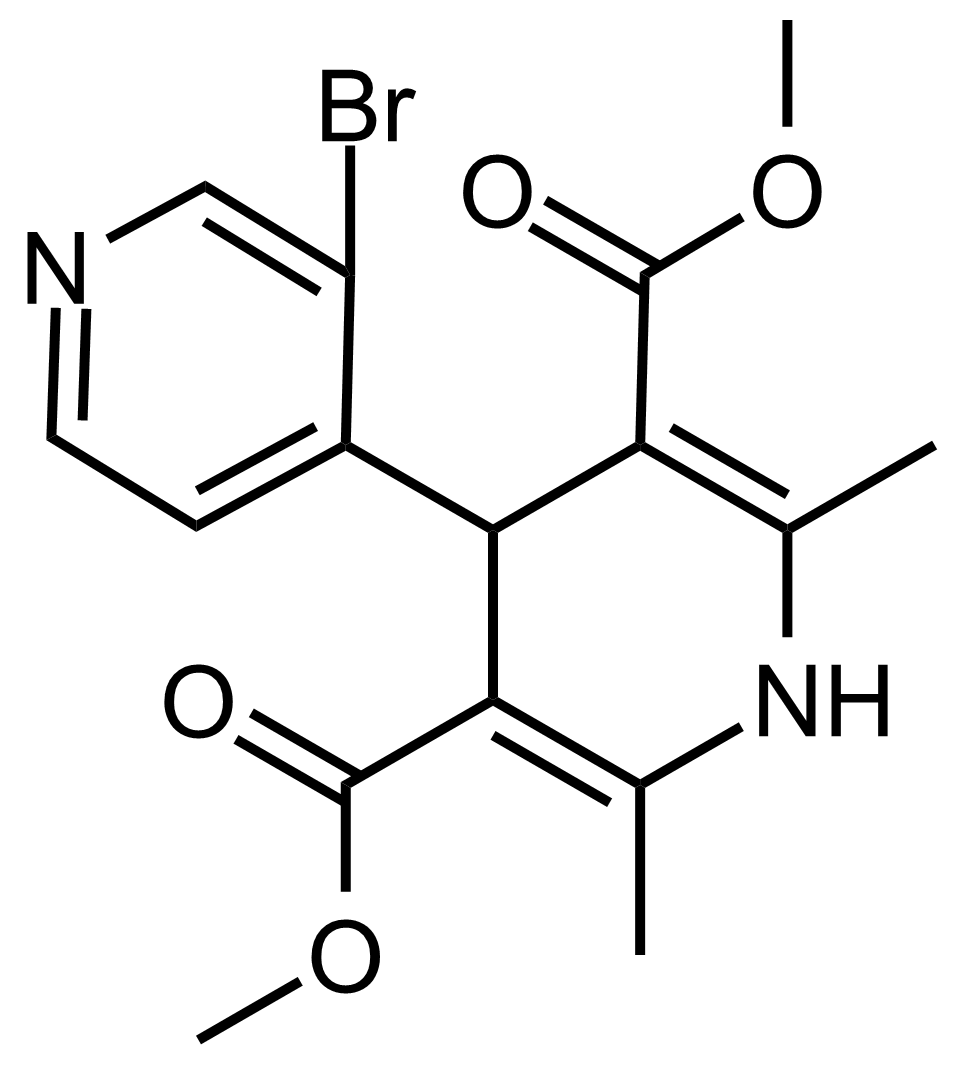 | N/A | GEO-03686 |
| Dimethyl 6-bromo-2′,6′-dimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate | 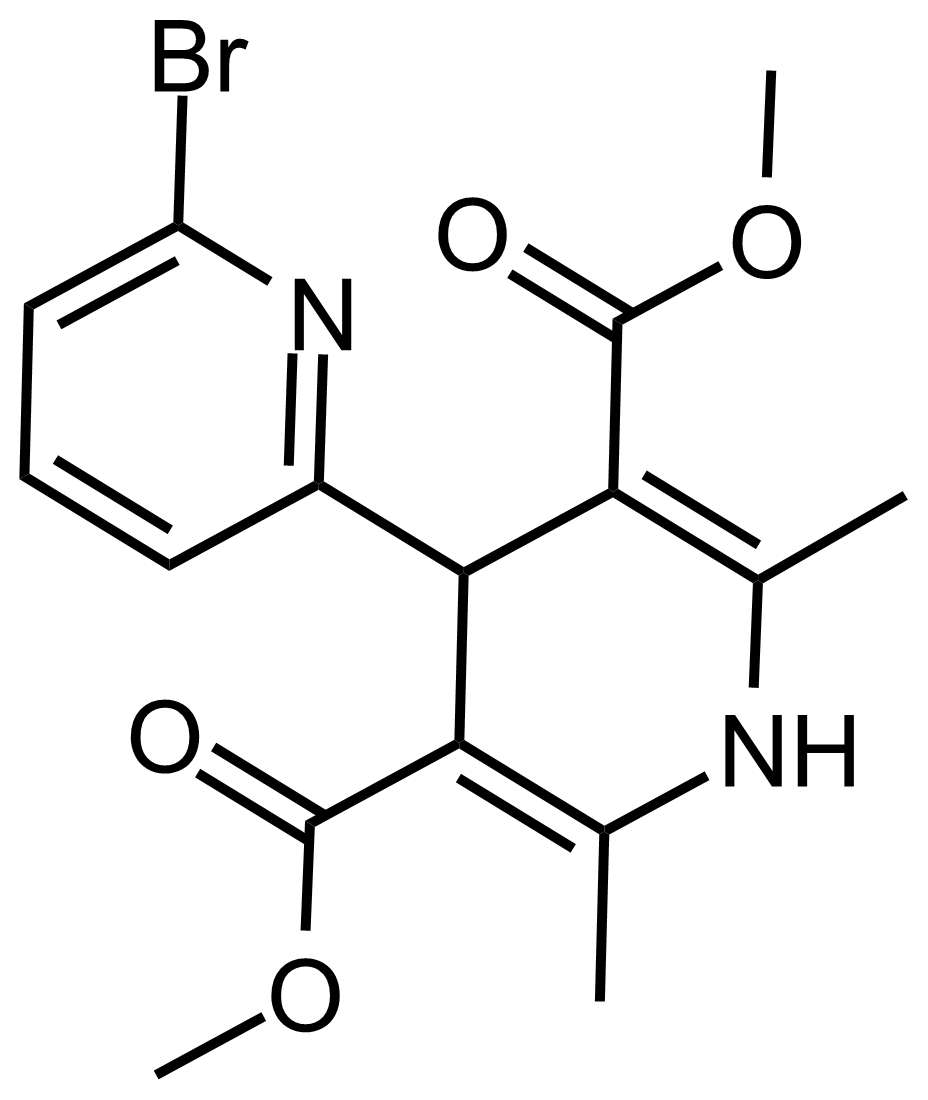 | N/A | GEO-03510 |
| Dimethyl 4-(5-bromo-2-fluorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 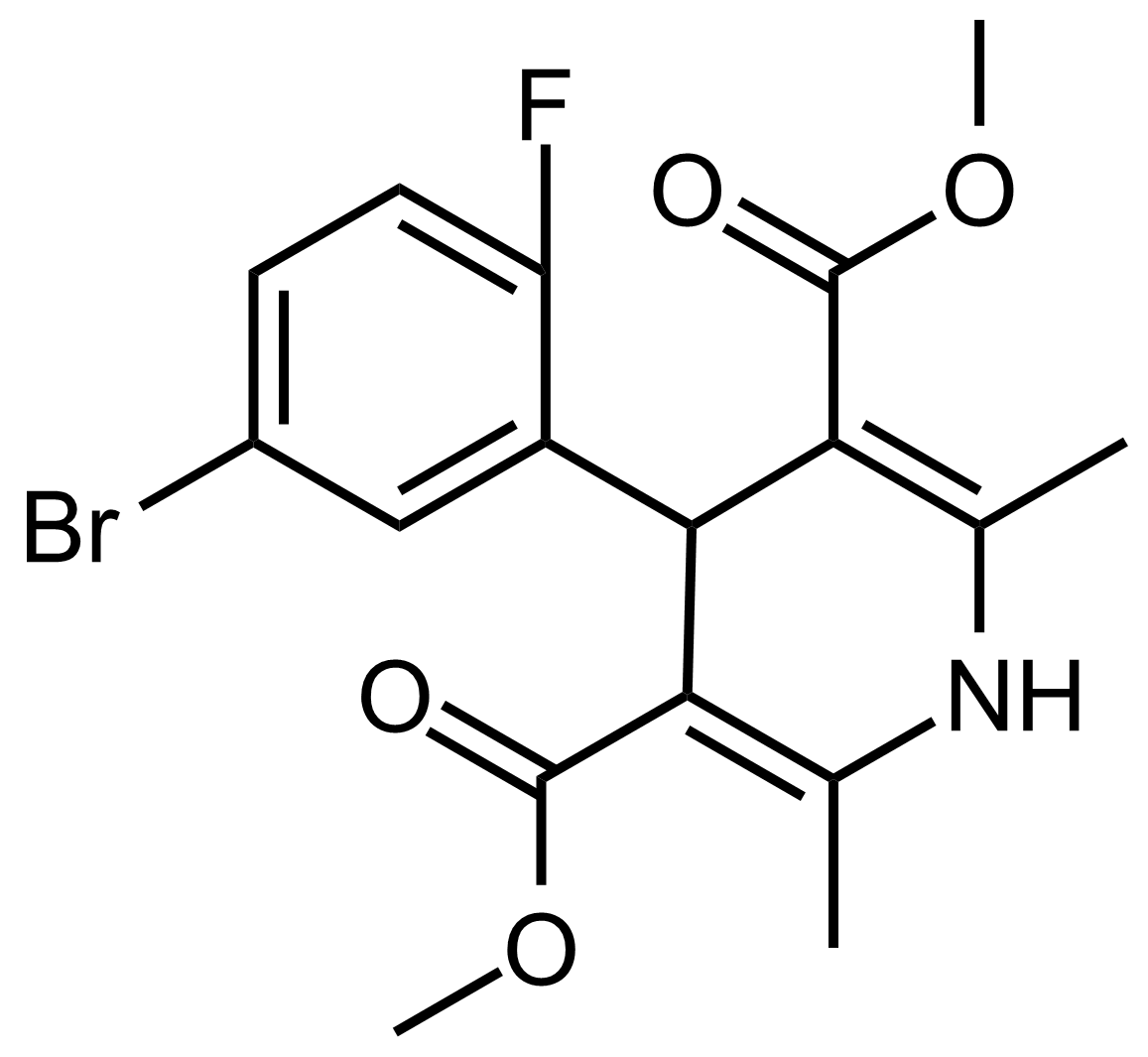 | [] | GEO-03621 |
| Dimethyl 4-(5-(4-bromo-2-fluorophenyl)furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 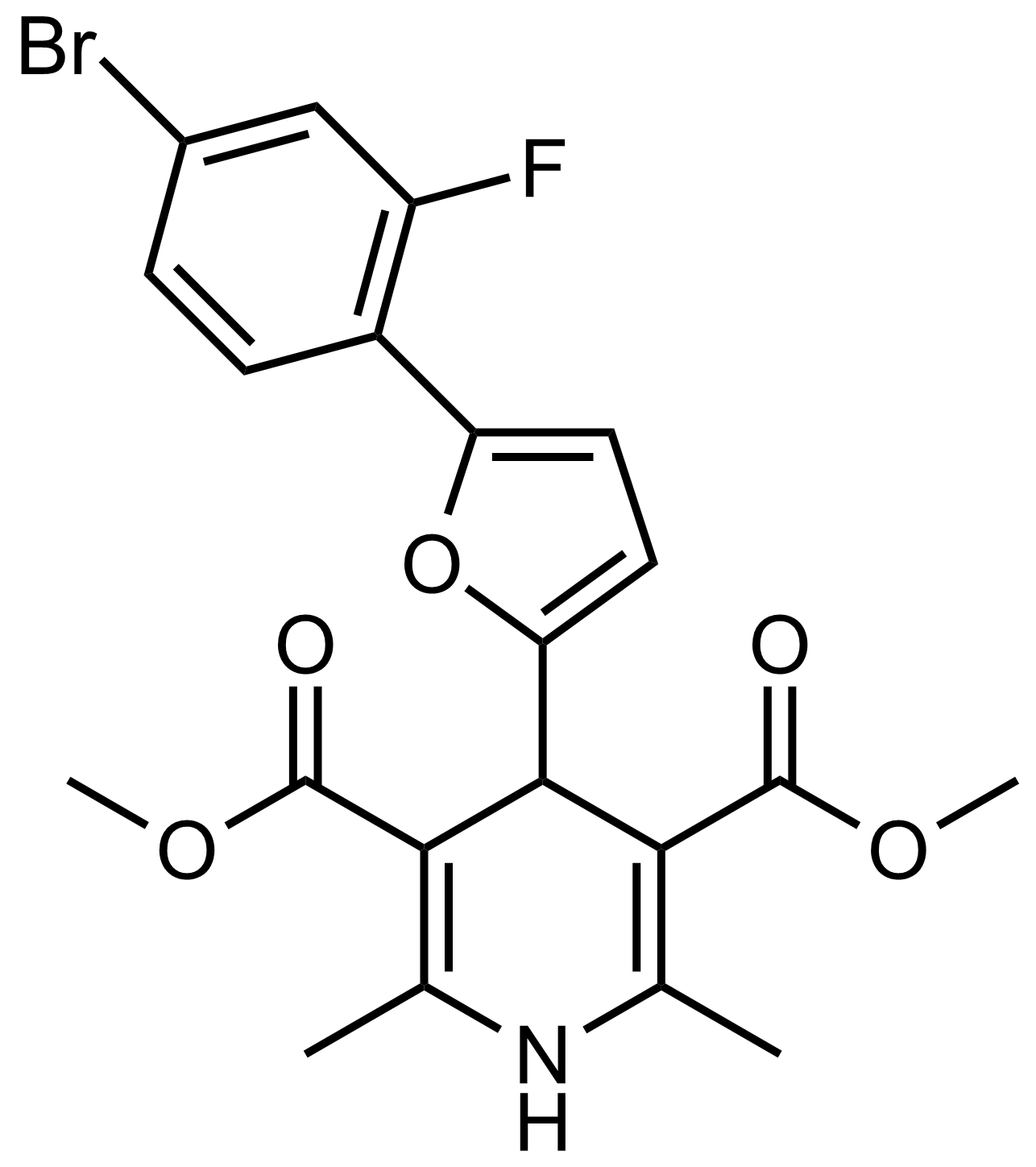 | [] | GEO-03639 |
| Dimethyl 4-(4-bromothiophen-3-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 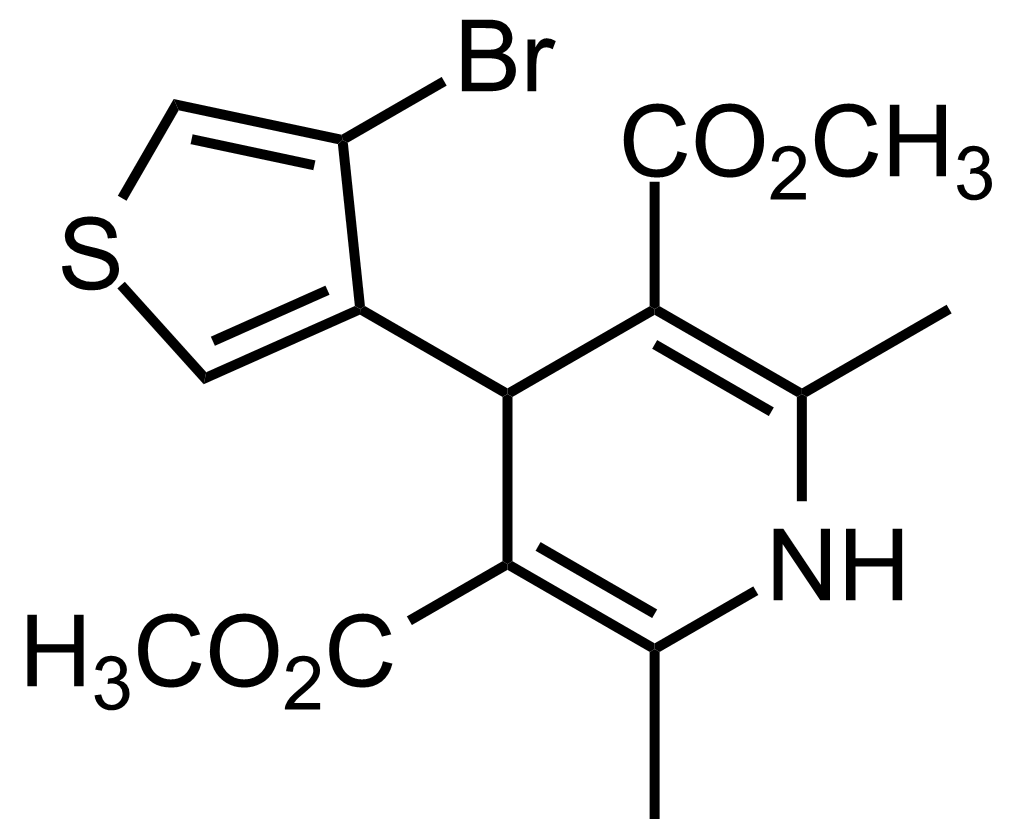 | [] | GEO-03660 |
| Dimethyl 4-(4-(tert-butyl)phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 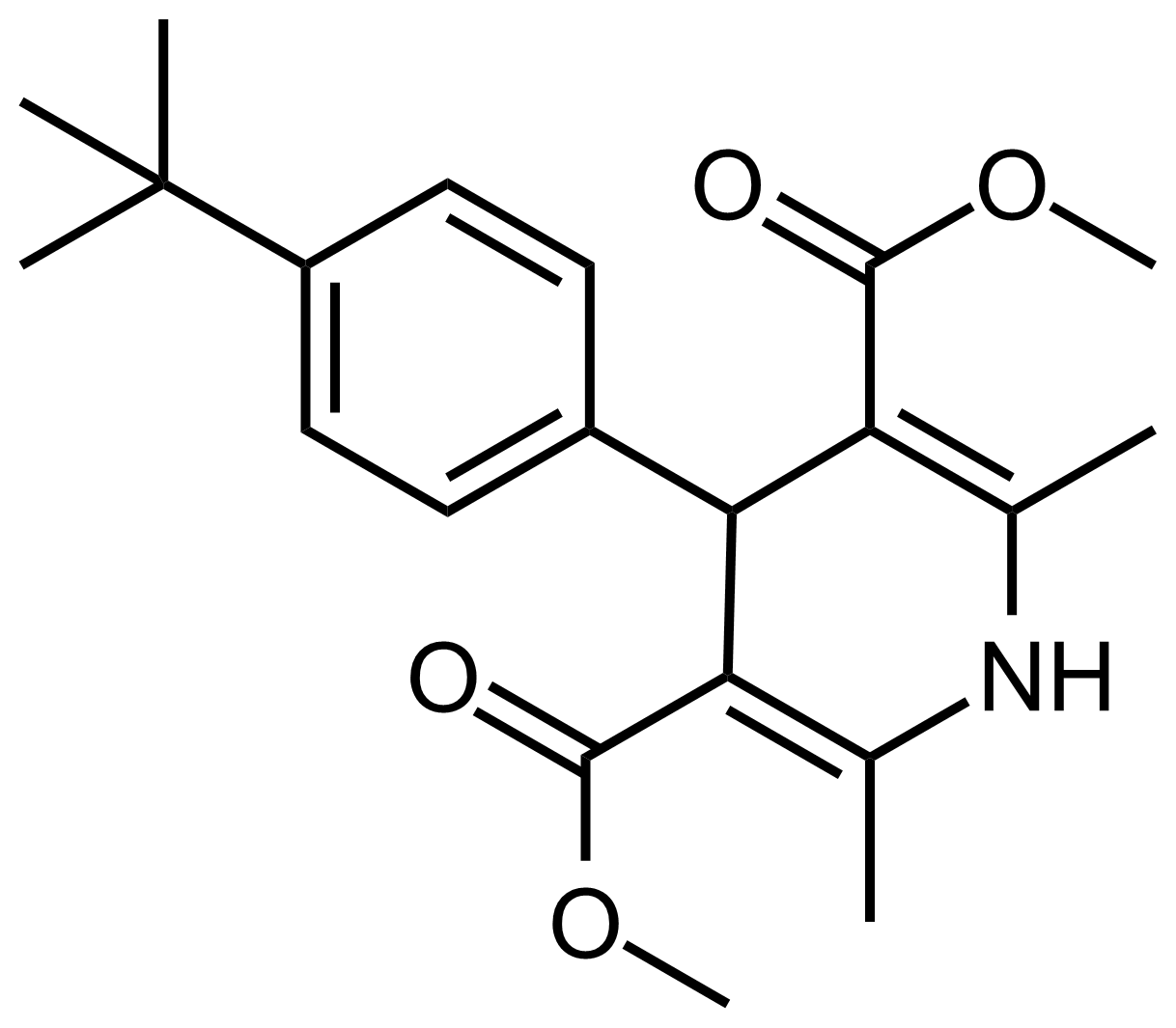 | N/A | GEO-03607 |
| Dimethyl 2′-chloro-2,6-dimethyl-1,4-dihydro-[4,4′-bipyridine]-3,5-dicarboxylate | 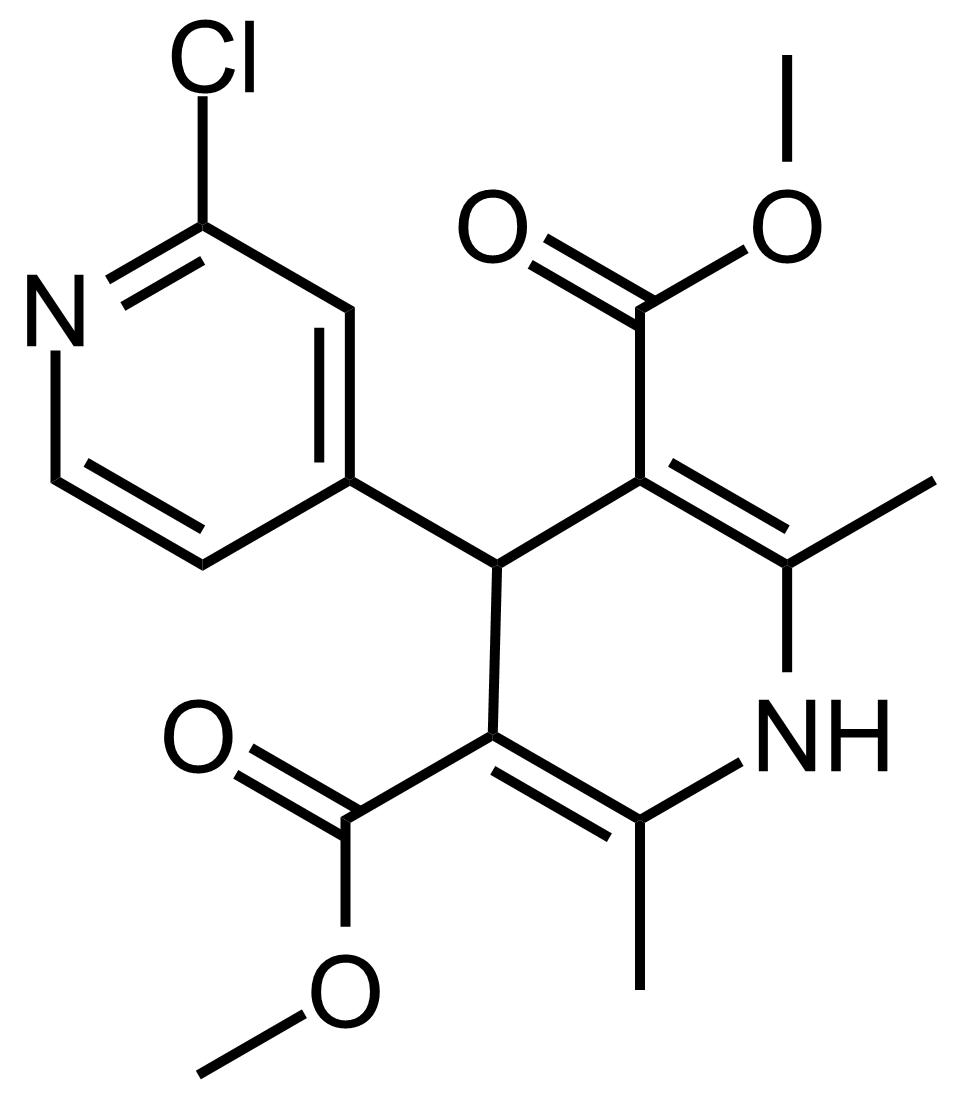 | N/A | GEO-03578 |
| Dimethyl 4-(3-chloro-4-methoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate |  | N/A | GEO-03612 |
| Dimethyl 4-(5-(3-chloro-4-methoxyphenyl)furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate |  | [] | GEO-03646 |
| Dimethyl-2,5-diaminothiophene-3,4-dicarboxylate |  | N/A | GEO-01170 |
| Dimethyl 2,4-dibromoglutarate | 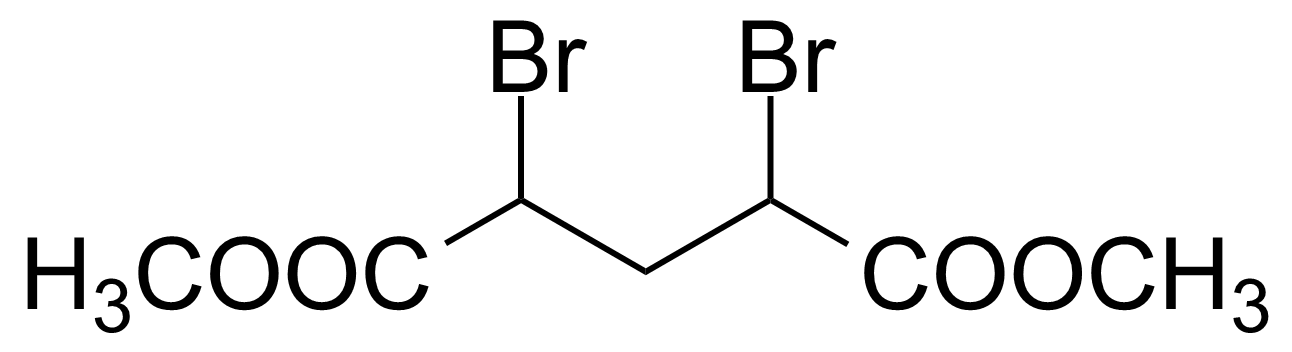 | [869-09-0] | GEO-01171 |
| Dimethyl 2,4-dichloro-2′,6′-dimethyl-1′,4′-dihydro-[3,4′-bipyridine]-3′,5′-dicarboxylate |  | N/A | GEO-03606 |
| Dimethyl 2,6-dichloro-2′,6′-dimethyl-1′,4′-dihydro-[3,4′-bipyridine]-3′,5′-dicarboxylate | 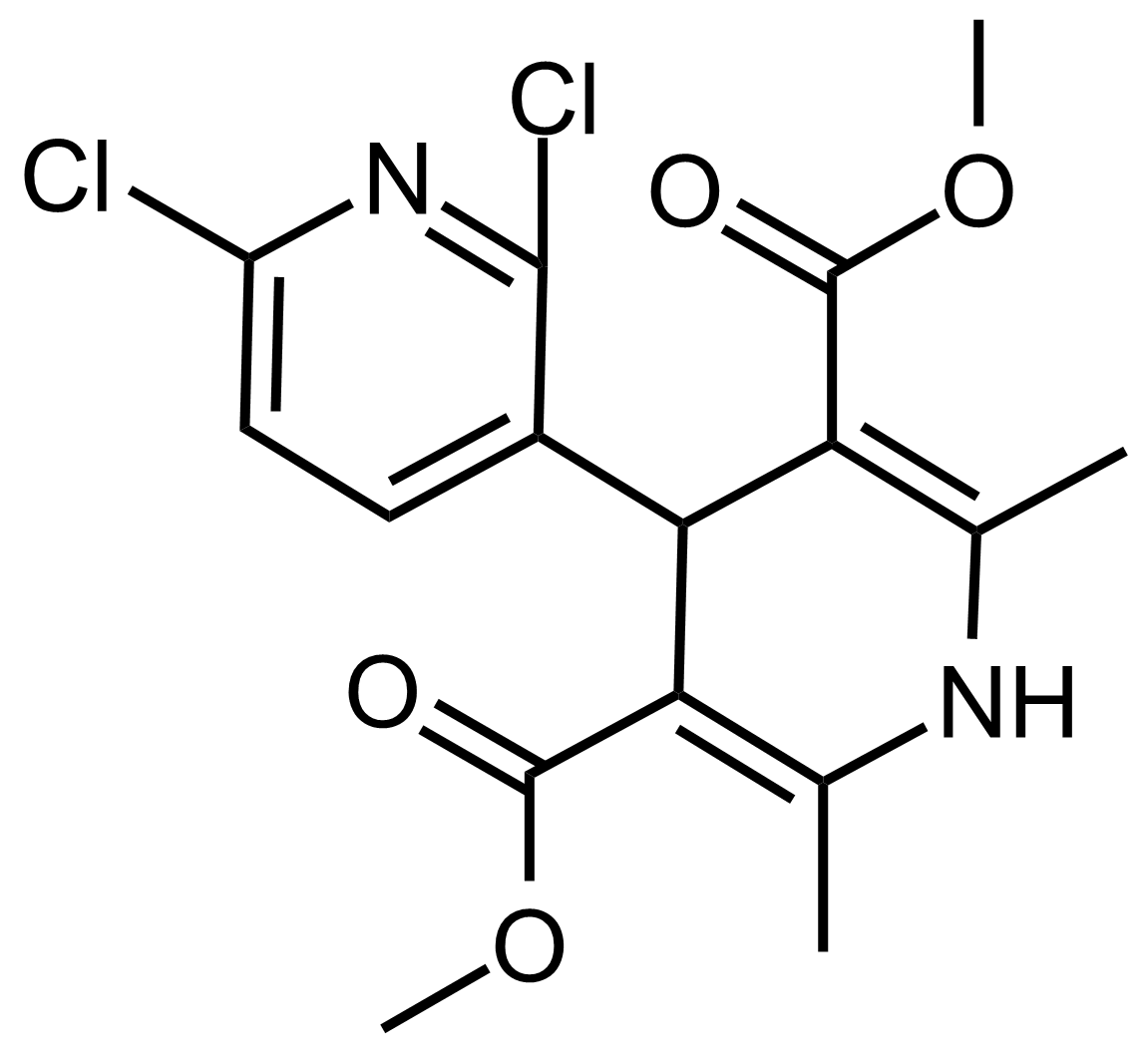 | N/A | GEO-03518 |
| Dimethyl 4-(2,6-difluoro-4-methoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 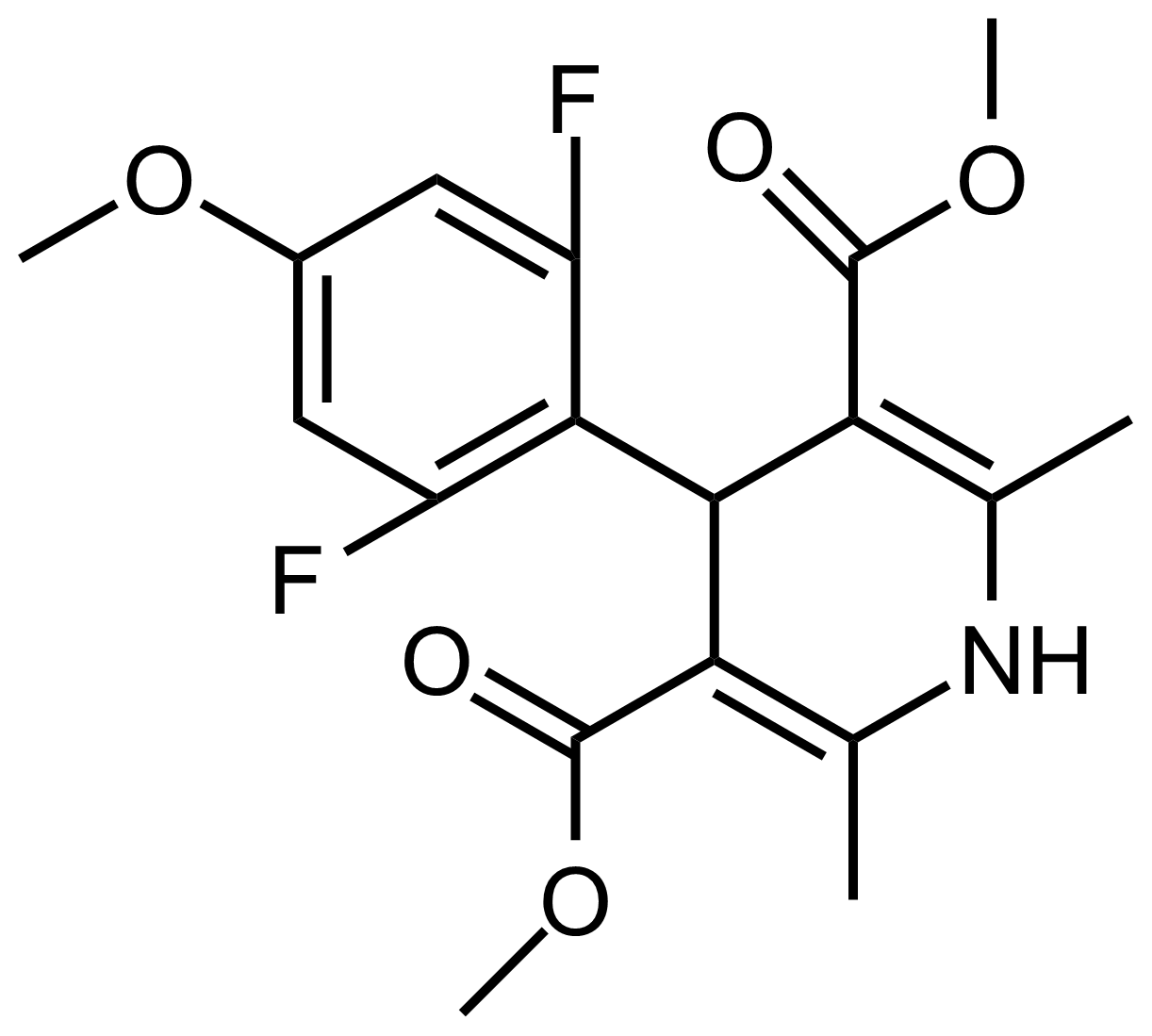 | N/A | GEO-03503 |
| Dimethyl 2,5-dihydrothiophene-3,4-dicarboxylate | 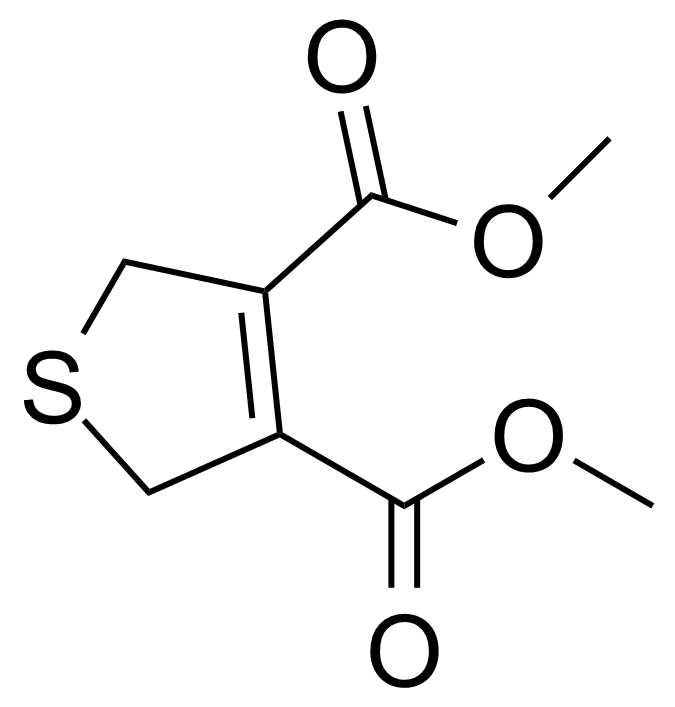 | [20946-32-1] | GEO-04024 |
| Dimethyl 2′,6′-dimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate | 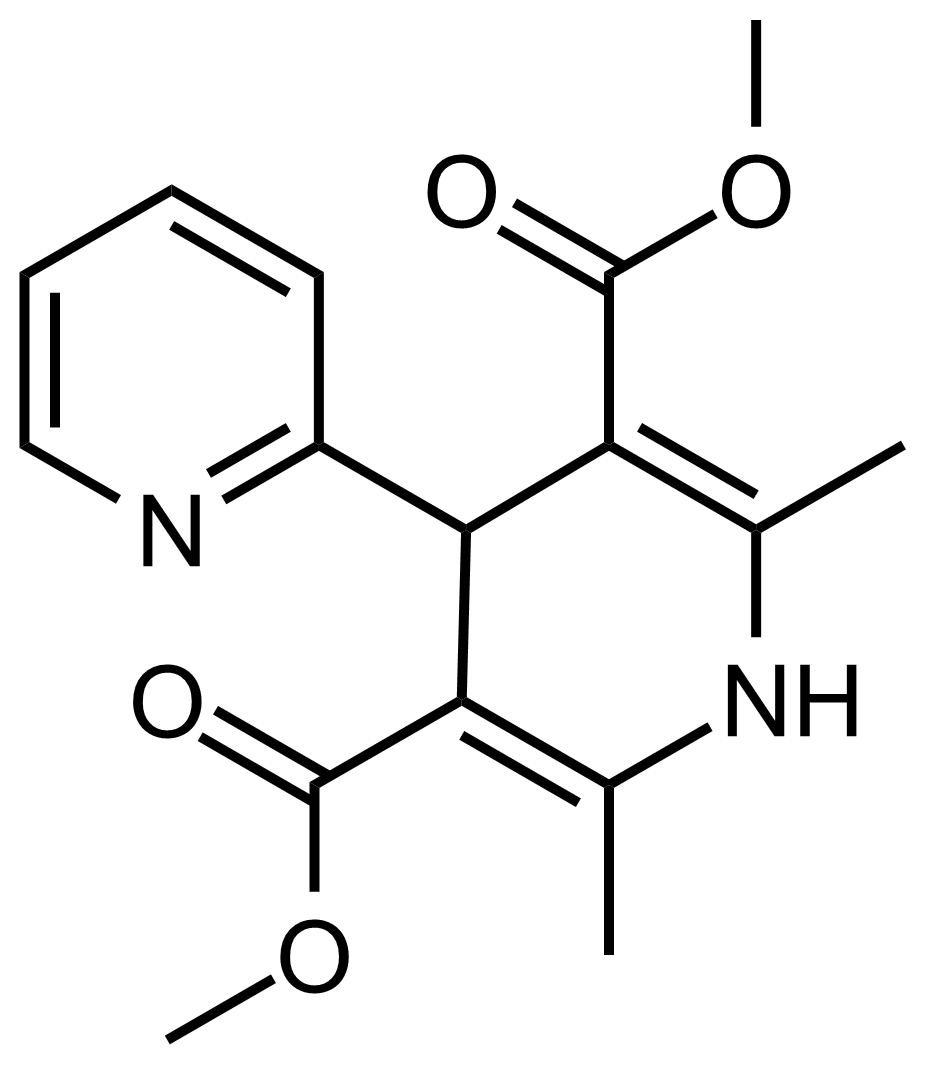 | N/A | GEO-03602 |
| Dimethyl 2,6-dimethyl-4-(5-methylfuran-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 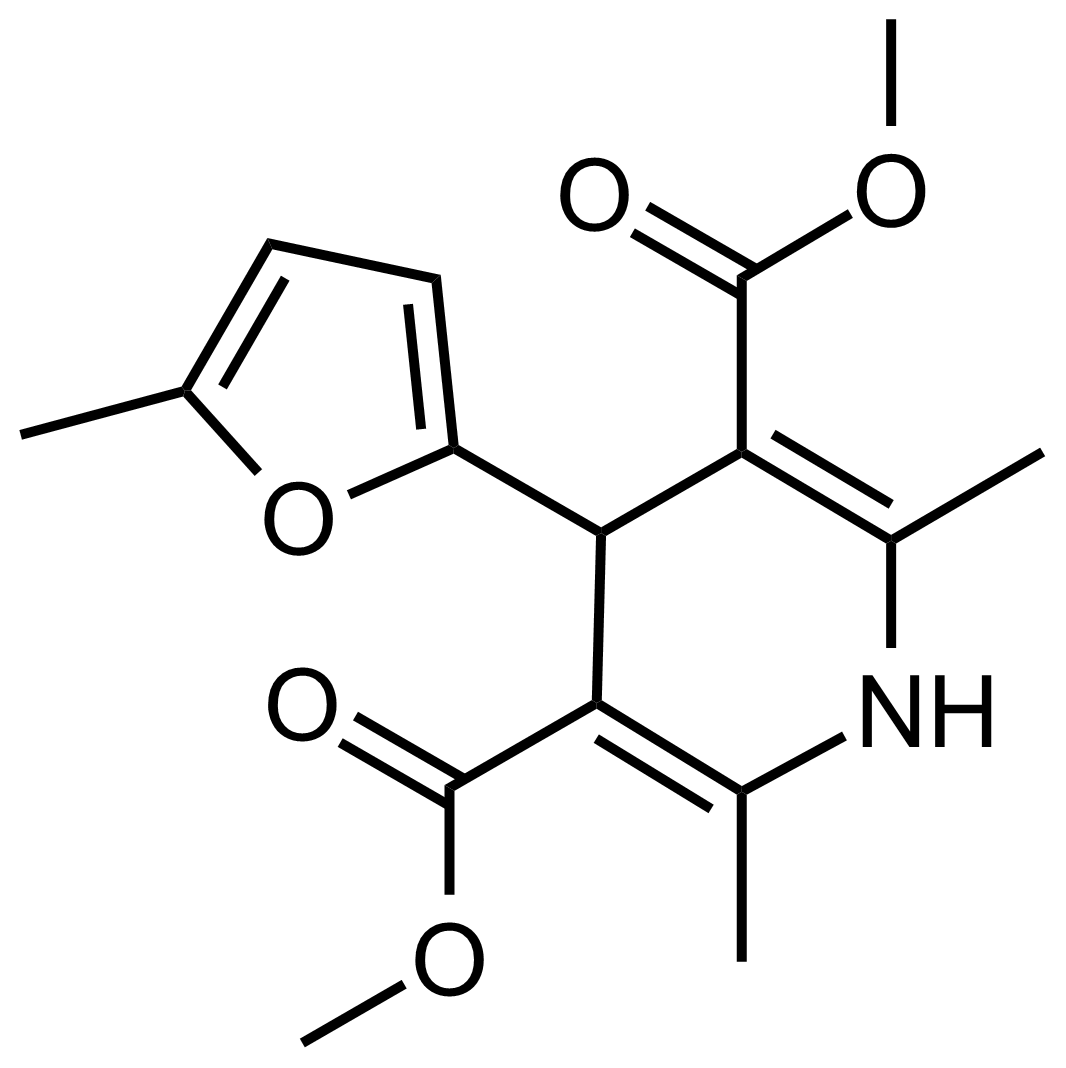 | N/A | GEO-03573 |
| Dimethyl 2,6-dimethyl-4-(5-((5-methyl-1,3,4-thiadiazol-2-yl)thio)furan-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 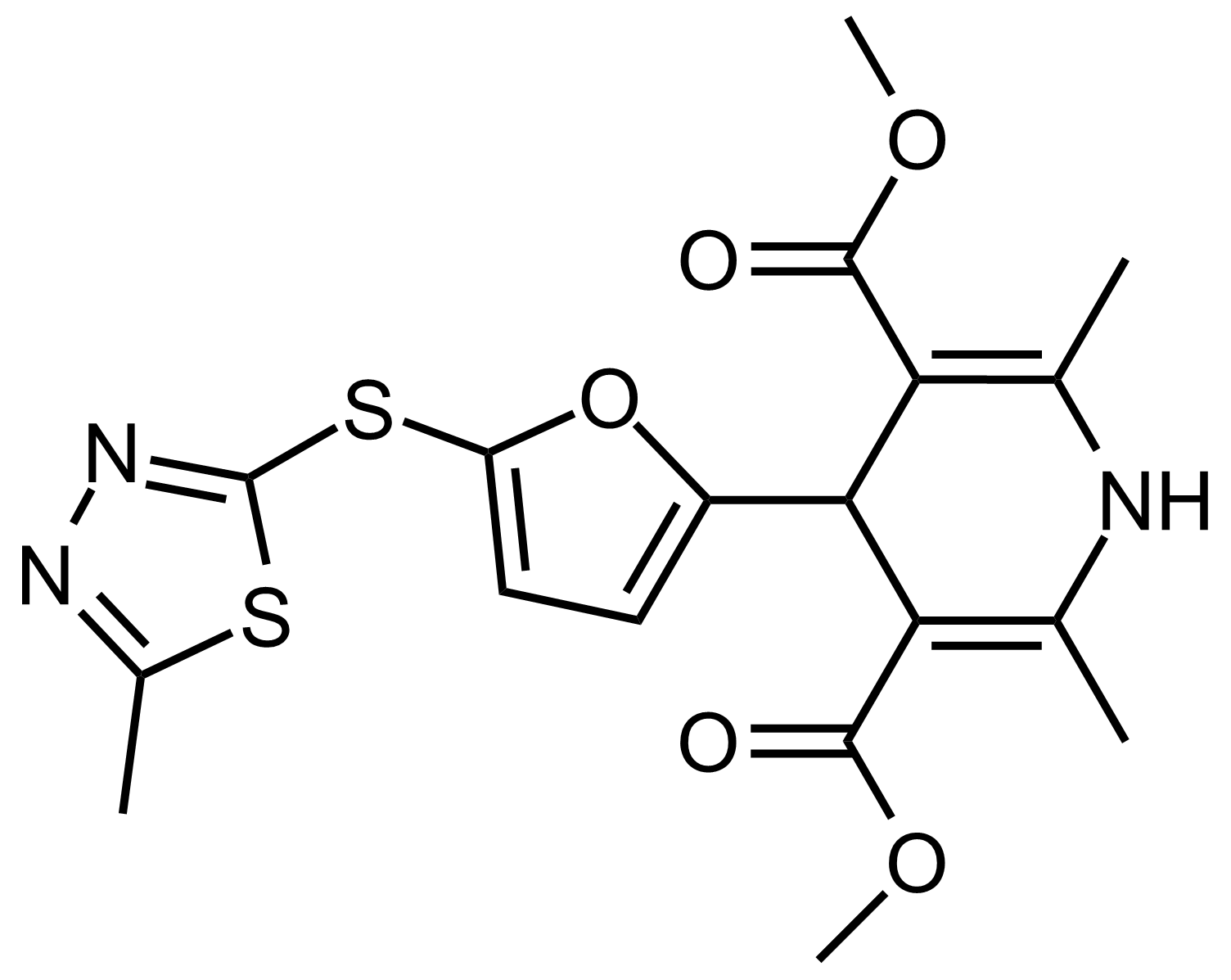 | N/A | GEO-03522 |
| Dimethyl 2,6-dimethyl-4-(5-(pyridin-2-ylthio)furan-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 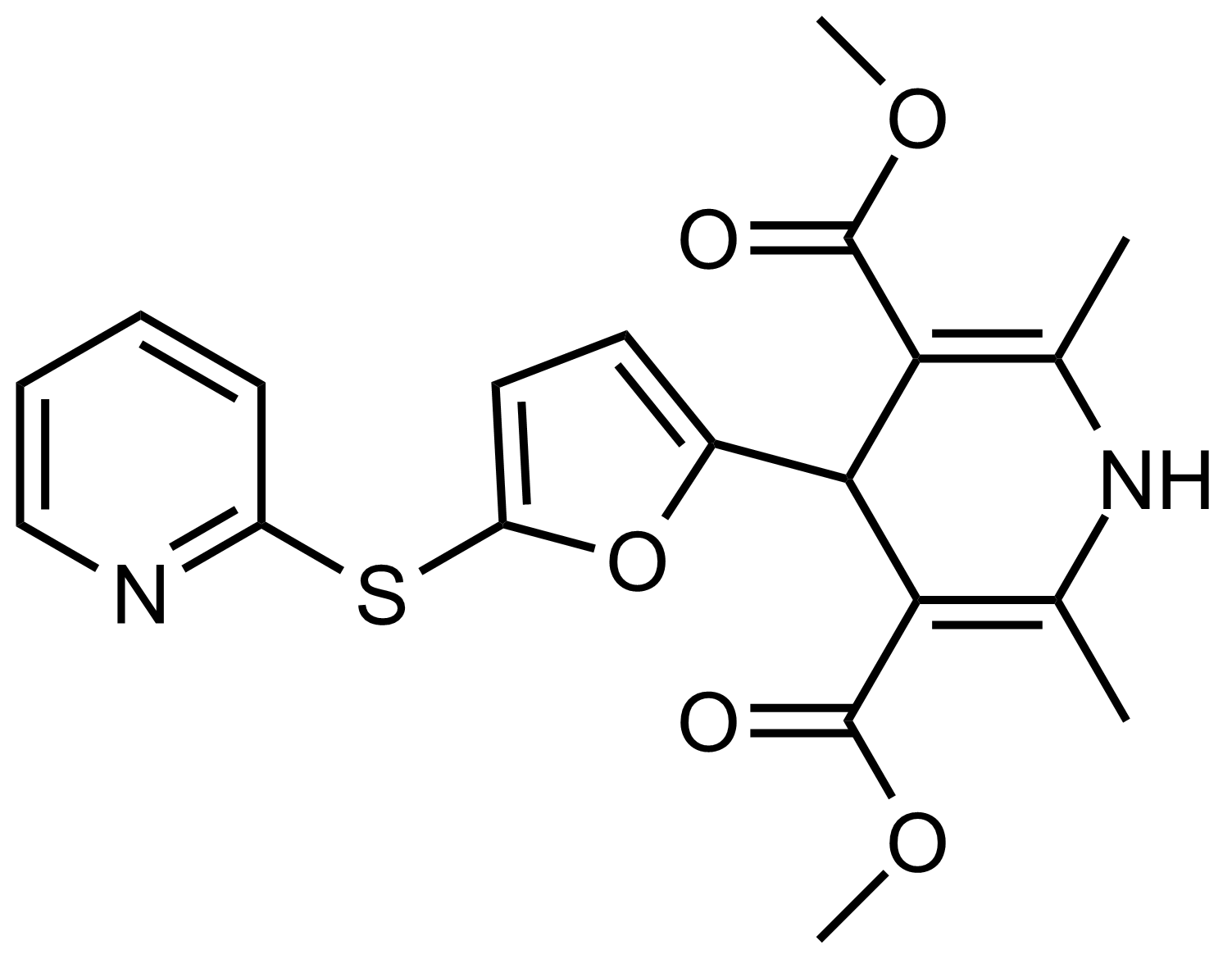 | N/A | GEO-03494 |
| Dimethyl 2,6-dimethyl-4-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-1,4-dihydropyridine-3,5-dicarboxylate | 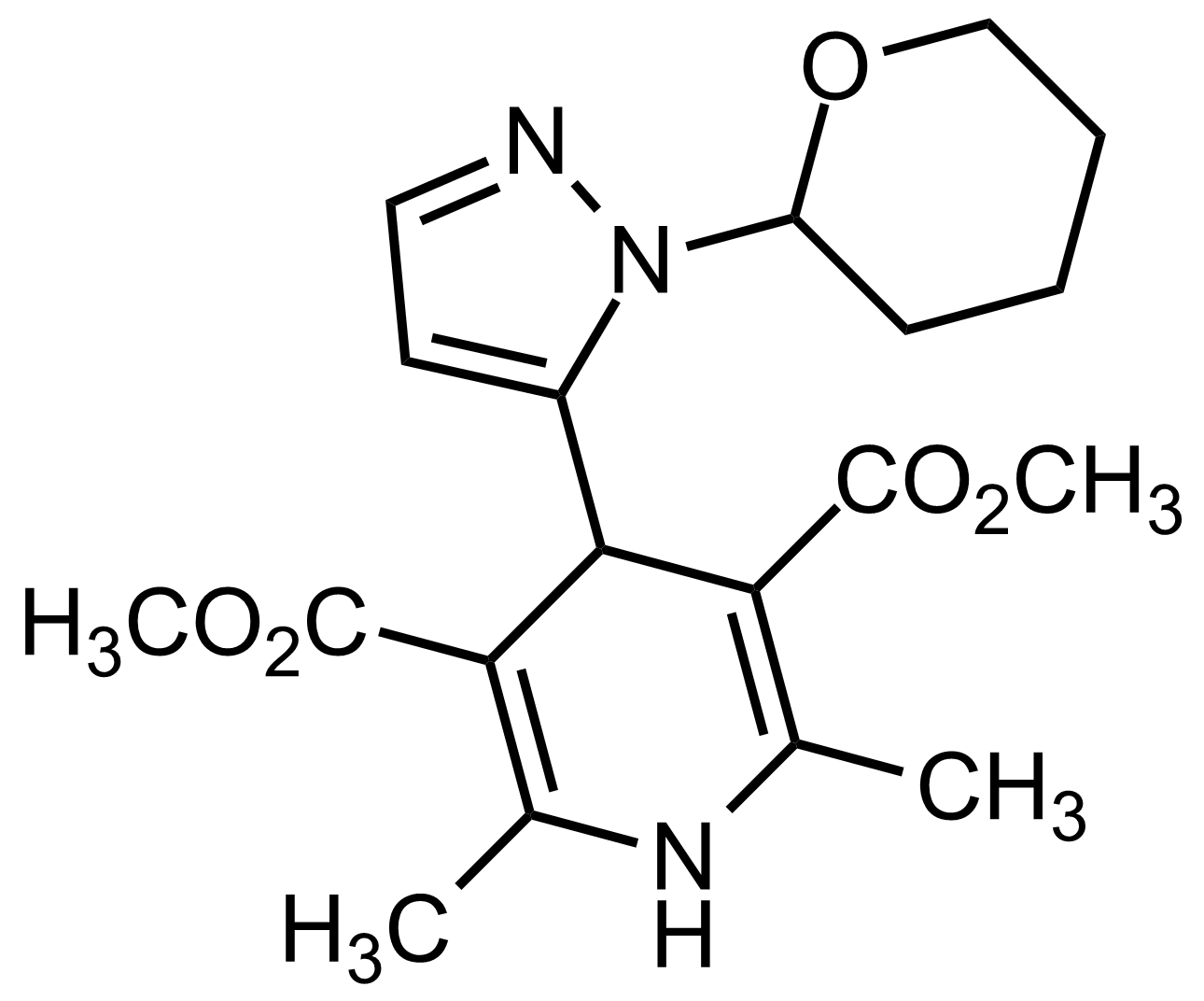 | N/A | GEO-03548 |
| Dimethyl 4-(4-ethoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate |  | N/A | GEO-03580 |
| Dimethyl 4-(3-fluorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 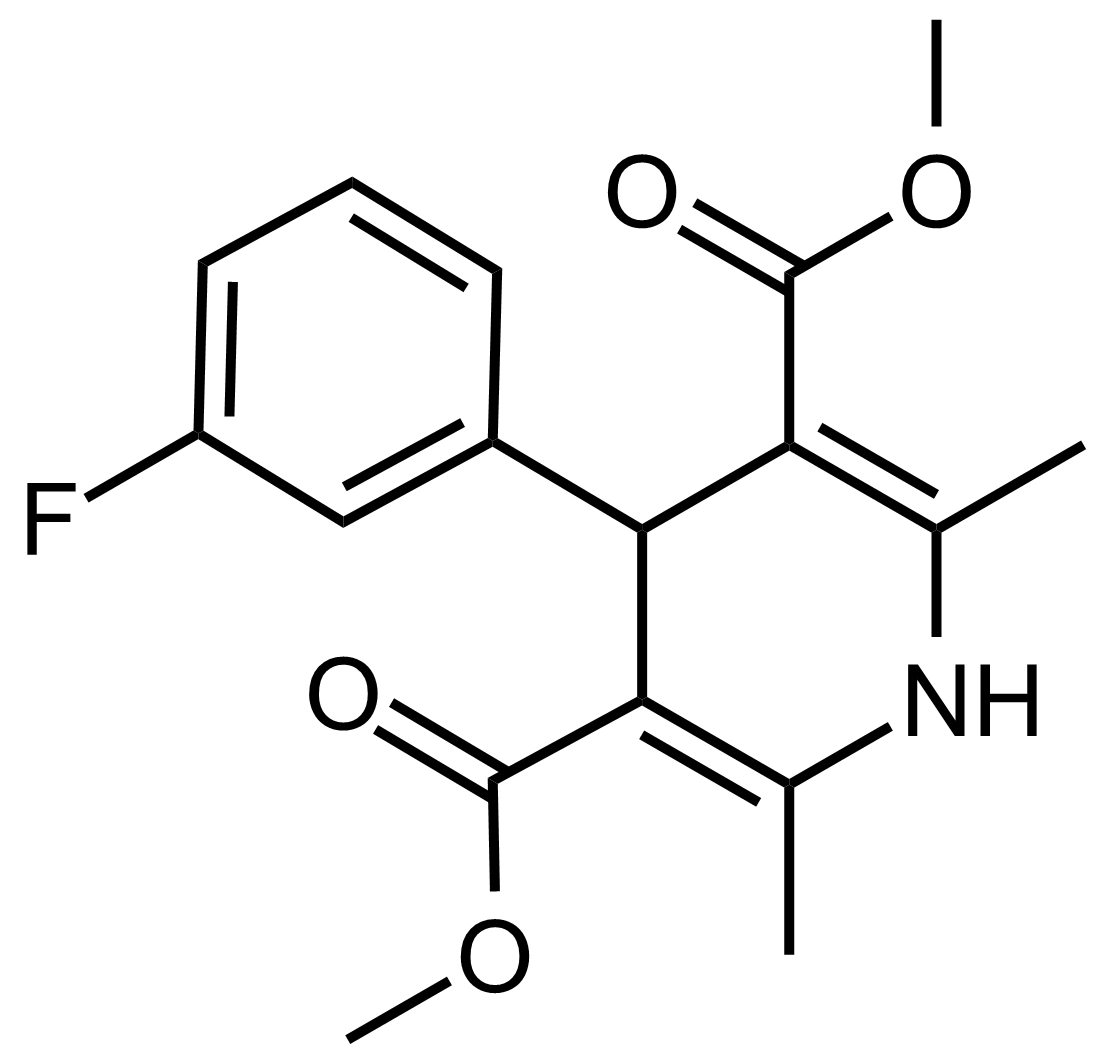 | N/A | GEO-03532 |
| Dimethyl 4-(4-fluorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 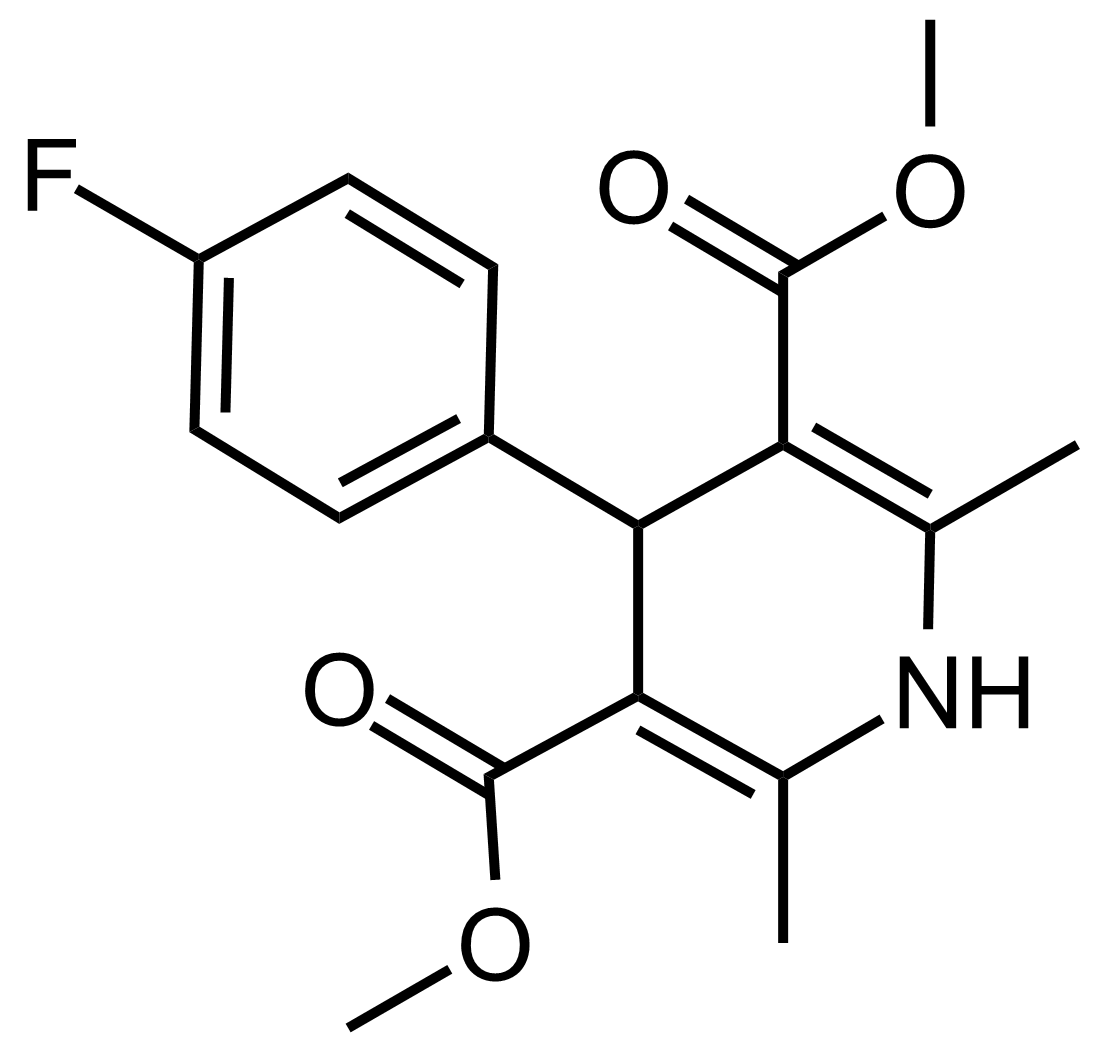 | N/A | GEO-03549 |
| Dimethyl 4-(5-hexylthiophen-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 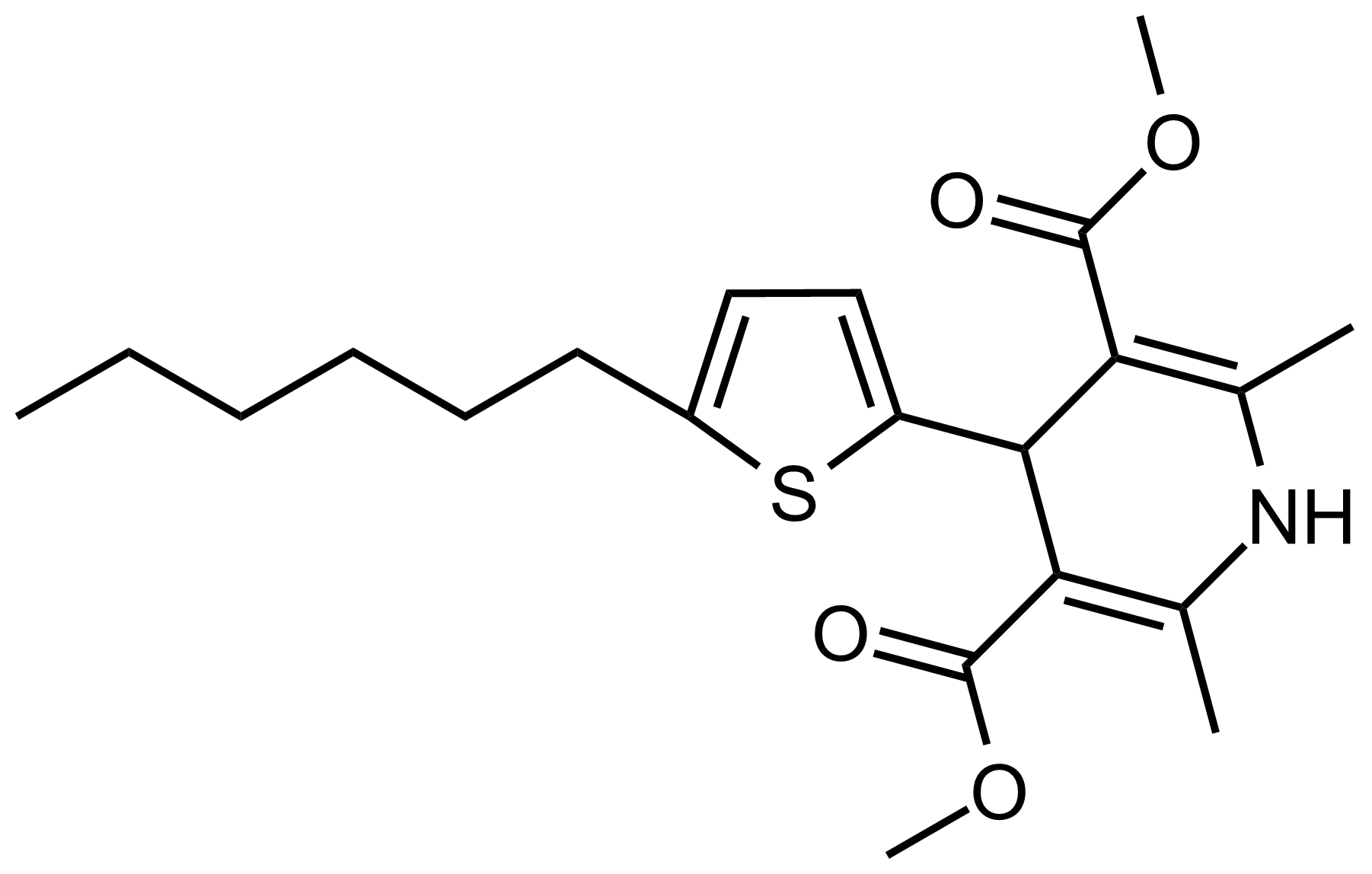 | N/A | GEO-03506 |
| Dimethyl 4-(1H-imidazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 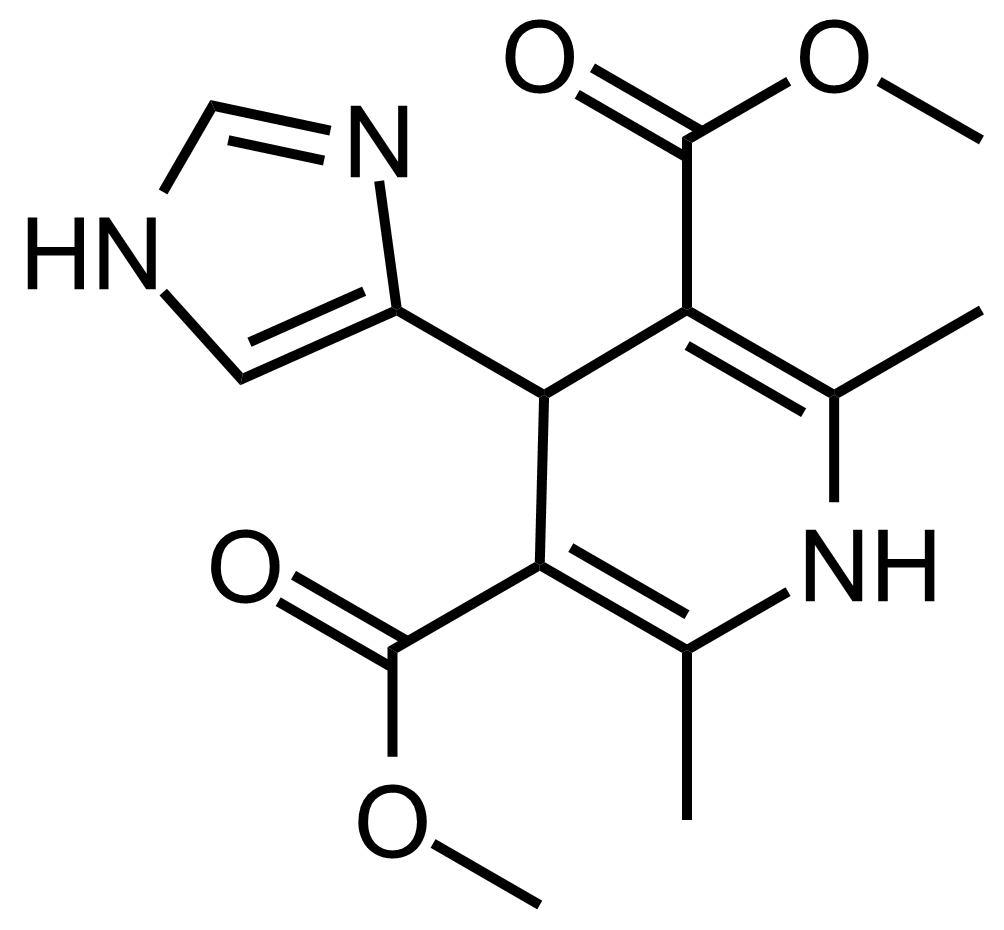 | [] | GEO-03641 |
| Dimethyl 4-isobutyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 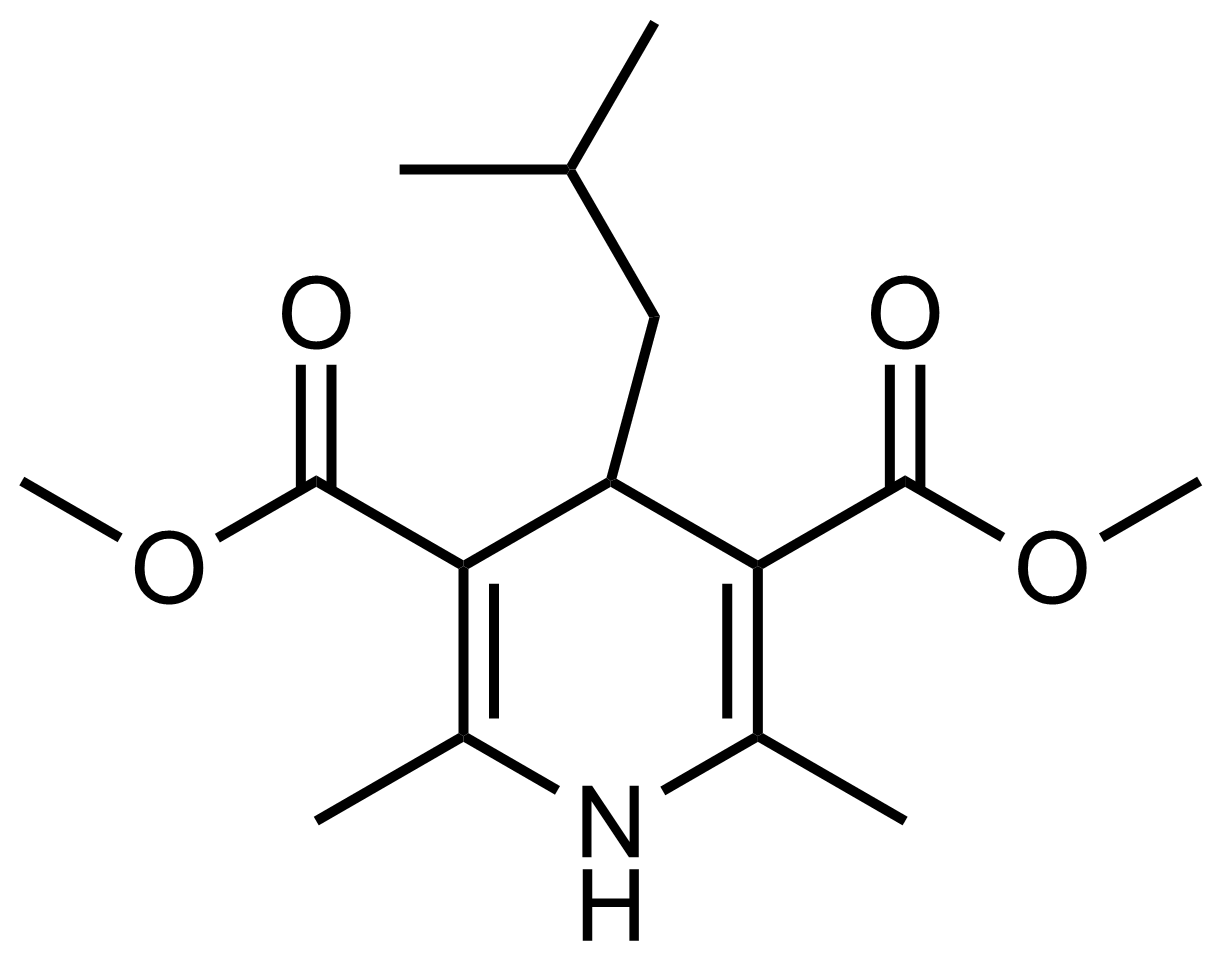 | [] | GEO-03629 |
| Dimethyl 4-isopropyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 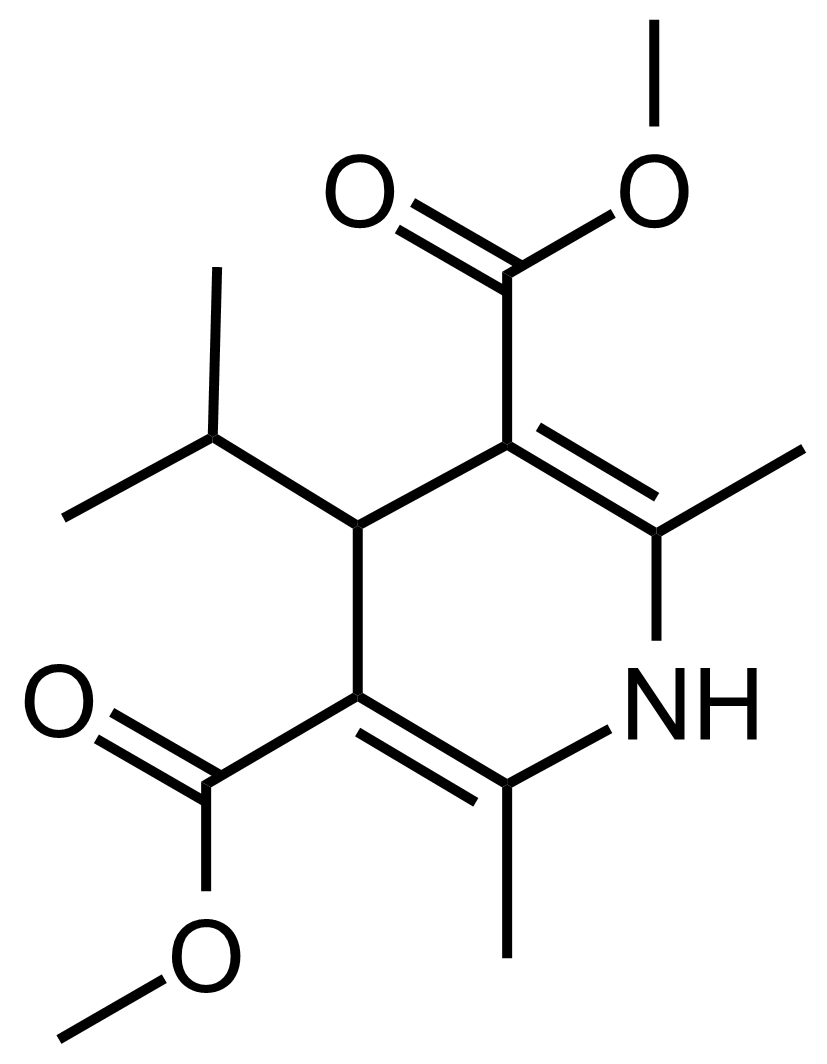 | N/A | GEO-03571 |
| Dimethyl 4-(2-methoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | 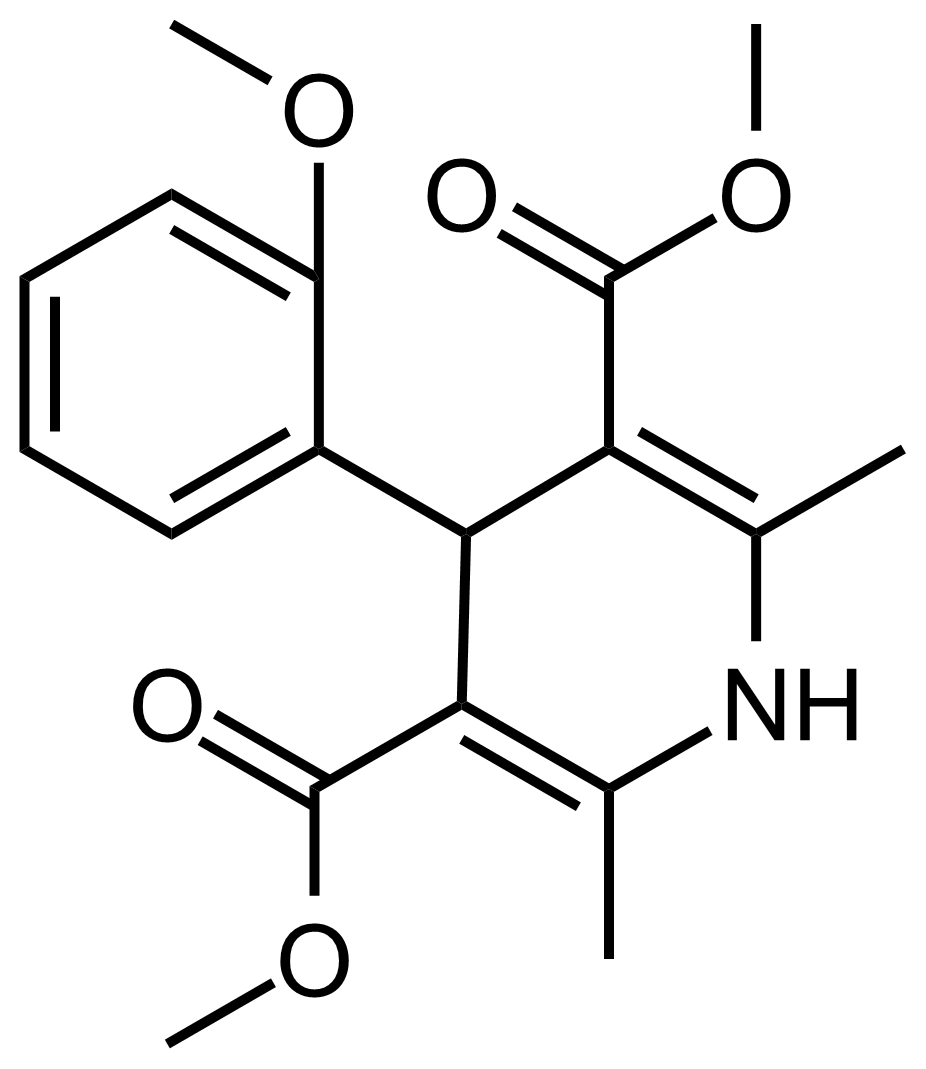 | N/A | GEO-03597 |
| Dimethyl 2,6-pyridinedicarboxylate |  | [5453-67-8] | GEO-01215 |
| Dimethyl 2′,6,6′-trimethyl-1′,4′-dihydro-[3,4′-bipyridine]-3′,5′-dicarboxylate | ![Structure of Dimethyl 2',6,6'-trimethyl-1',4'-dihydro-[3,4'-bipyridine]-3',5'-dicarboxylate](https://georganics.sk/wp-content/uploads/2021/06/GEO-03624_Dimethyl_266-trimethyl-14-dihydro-34-bipyridine-35-dicarboxylate.png) | [] | GEO-03624 |
| Dimethyl 2′,3,6′-trimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate |  | N/A | GEO-03492 |
| Dimethyl 2′,5,6′-trimethyl-1′,4′-dihydro-[2,4′-bipyridine]-3′,5′-dicarboxylate | 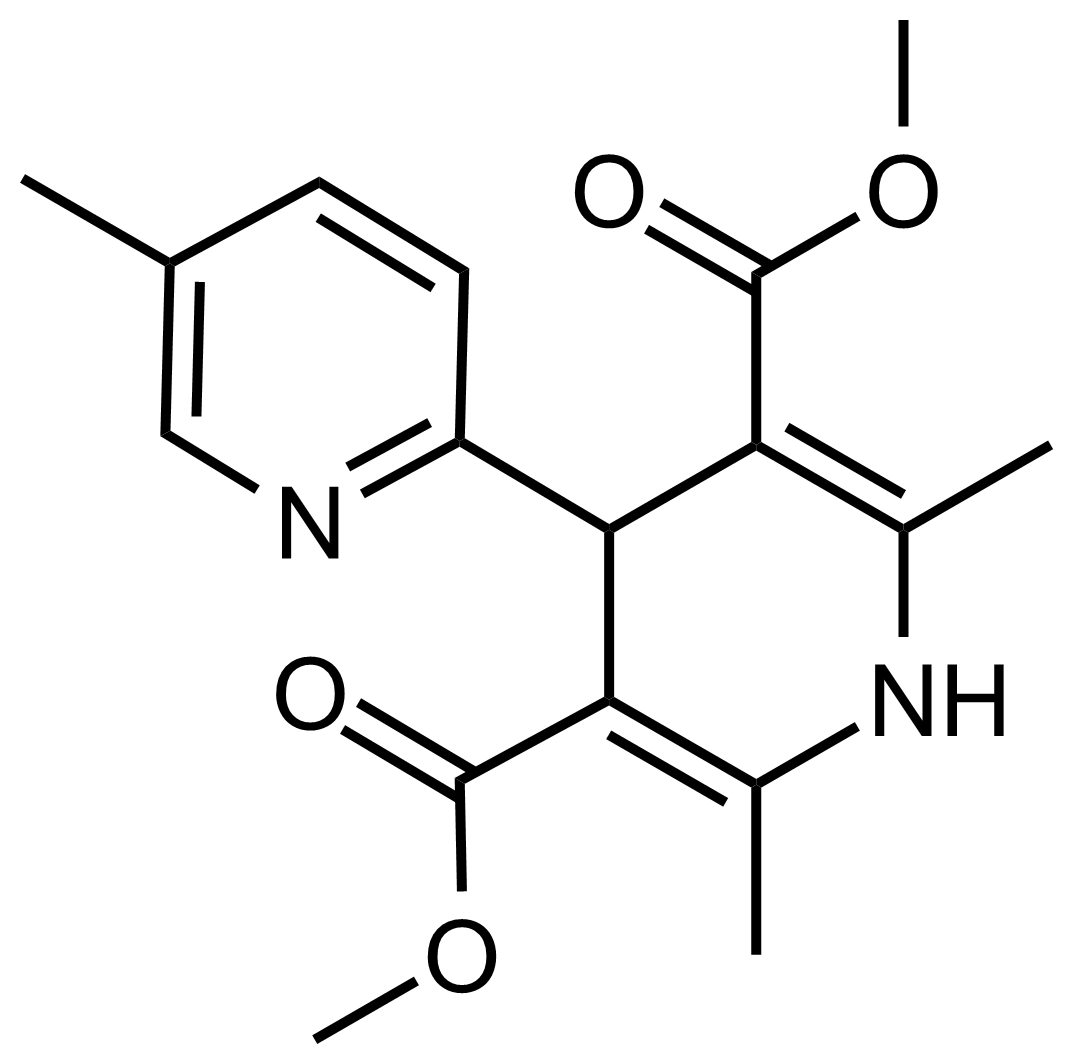 | N/A | GEO-03536 |
| Diphenylphosphonoacetic acid ethyl ester | 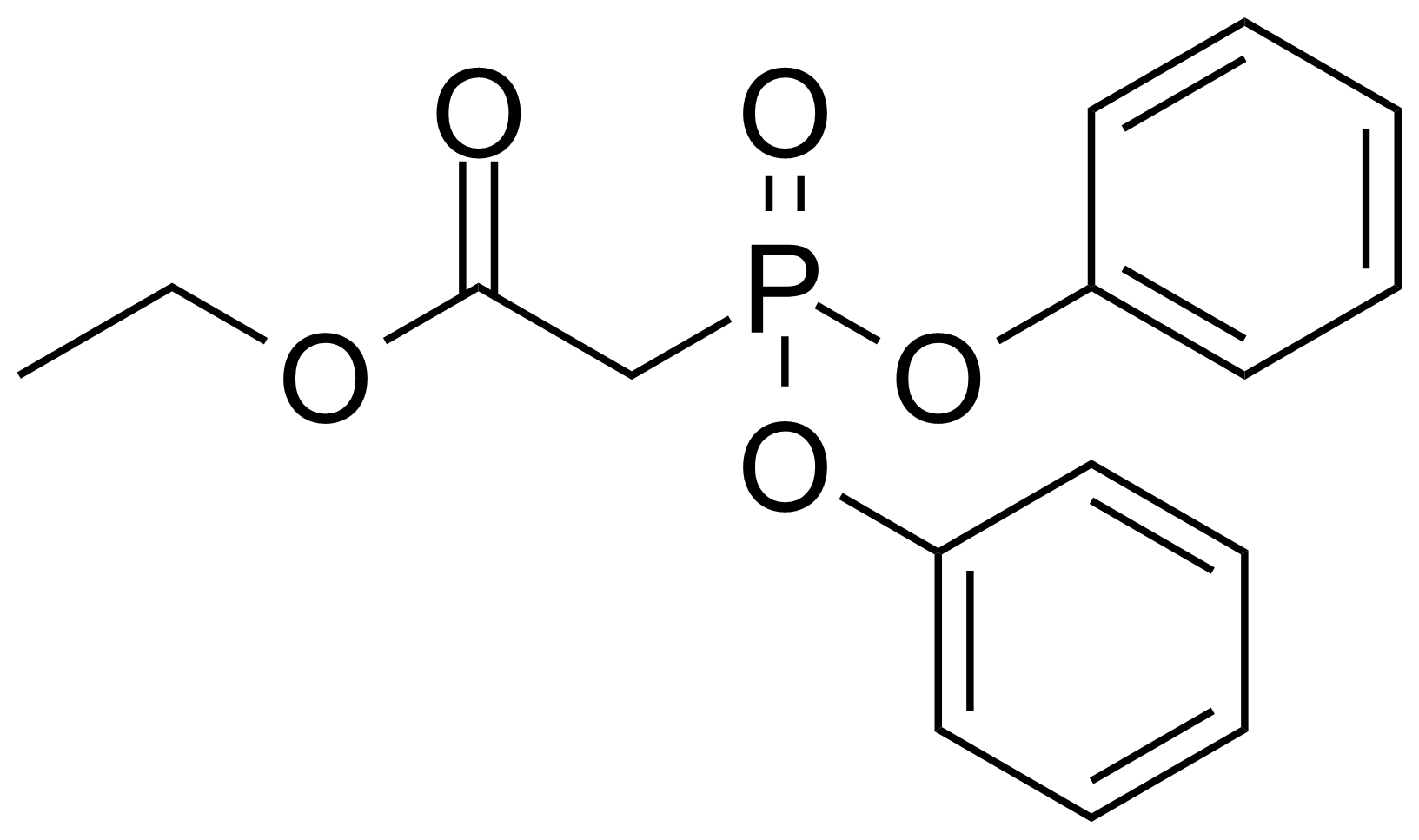 | [16139-79-0] | GEO-01243 |
| Ethyl 6-acetyl-2-pyridinecarboxylate | 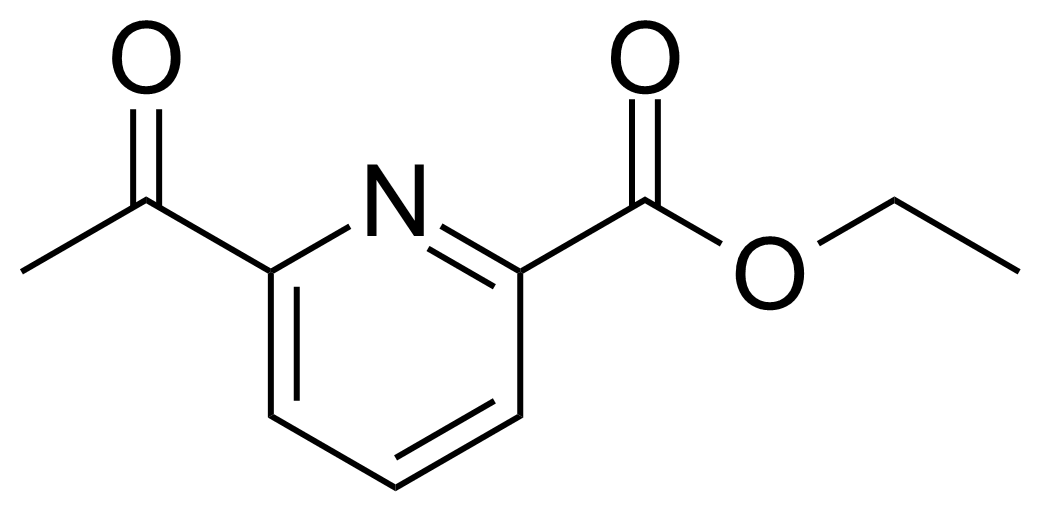 | [114578-70-0] | GEO-01309 |
| Ethyl 2-amino-1-cyclohexene-1-carboxylate | 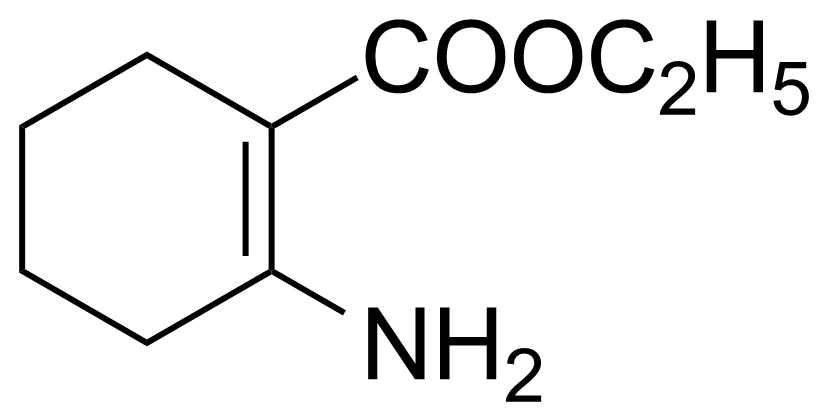 | [1128-00-3] | GEO-03438 |
| Ethyl 3-amino-5-methyl-1H-pyrazole-4-carboxylate | 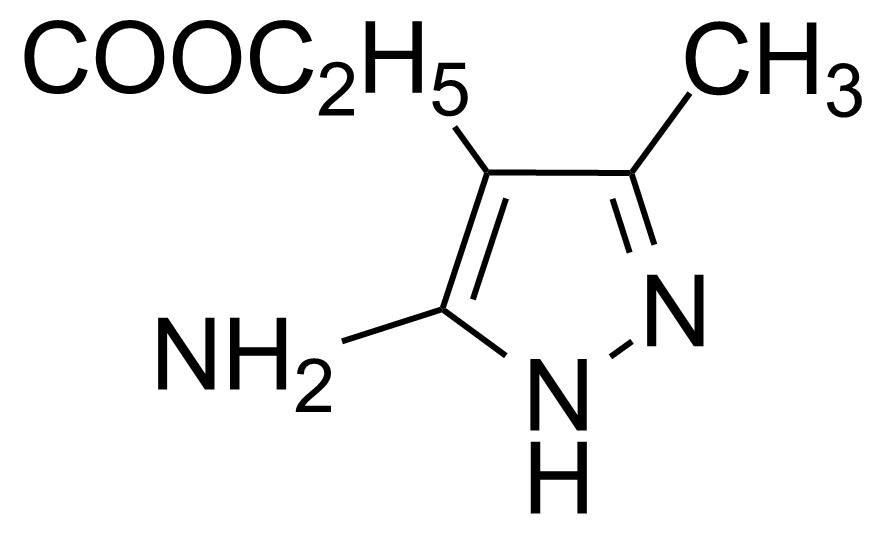 | [23286-70-6] | GEO-03006 |
| Ethyl 5-aminothiazole-4-carboxylate | 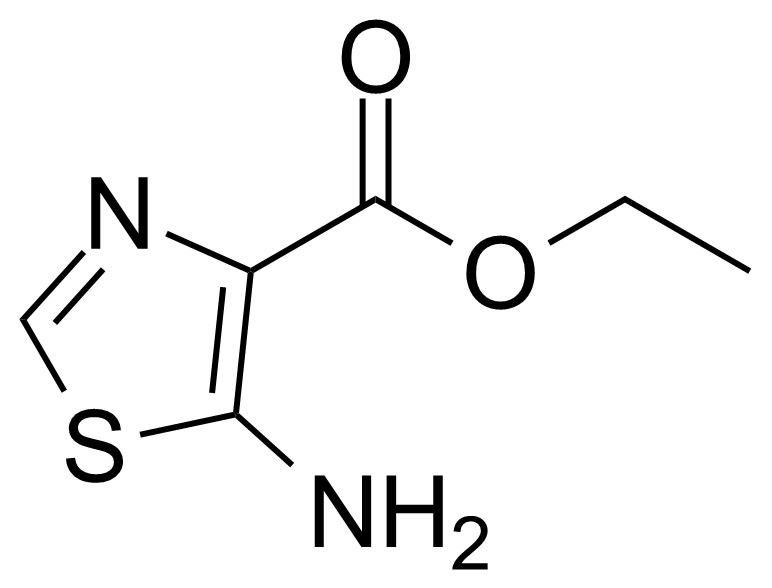 | [18903-18-9] | GEO-03986 |
| Ethyl 2-aminothiazole-5-carboxylate | 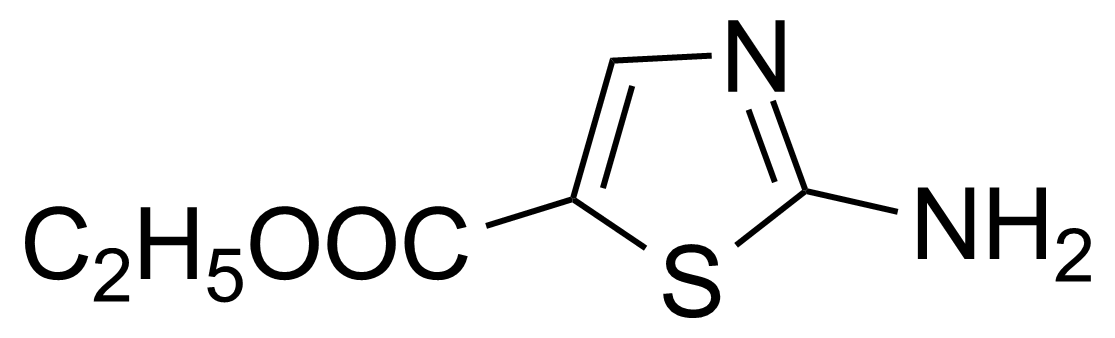 | [32955-21-8] | GEO-01317 |
| Ethyl 2-aminothiazole-4-carboxylate | 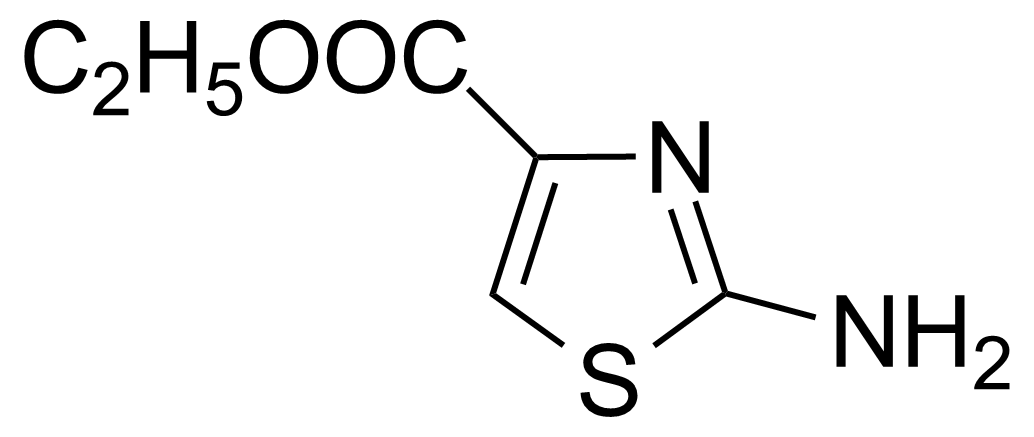 | [5398-36-7] | GEO-01316 |
| Ethyl 2-amino-4-trifluoromethyloxazole-5-carboxylate | 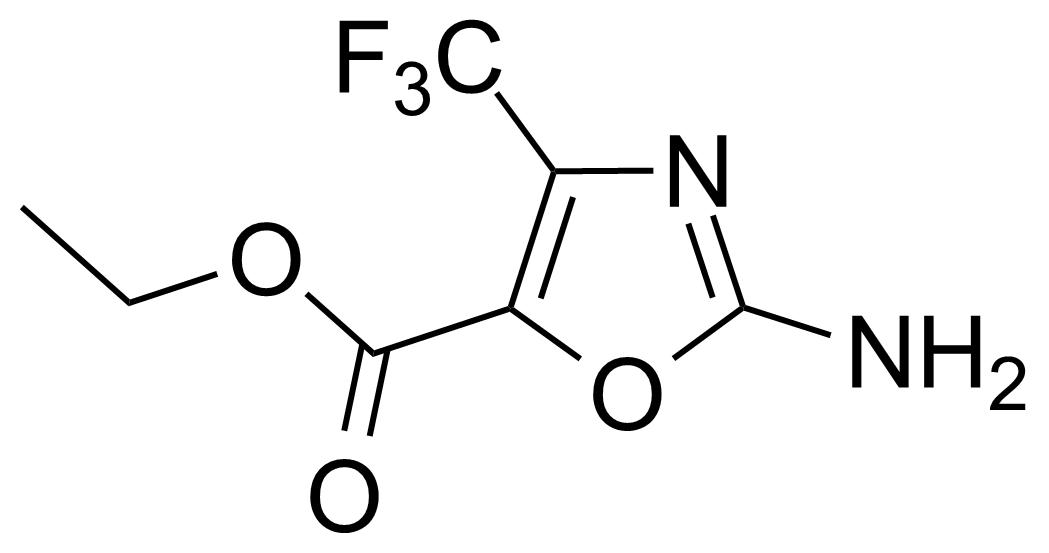 | [135026-17-4] | GEO-02774 |
| (E)-Ethyl 3-(3-bromopyridin-4-yl)-2-cyanoacrylate | 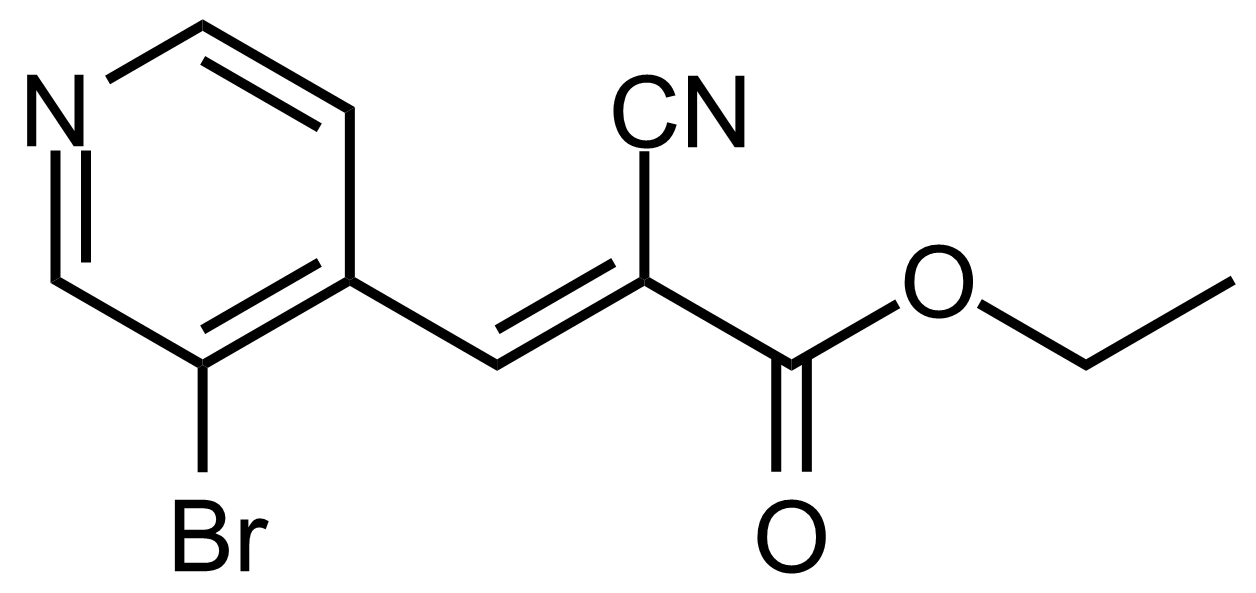 | [] | GEO-03649 |
| Ethyl 5-(chloromethyl)-2-furancarboxylate | 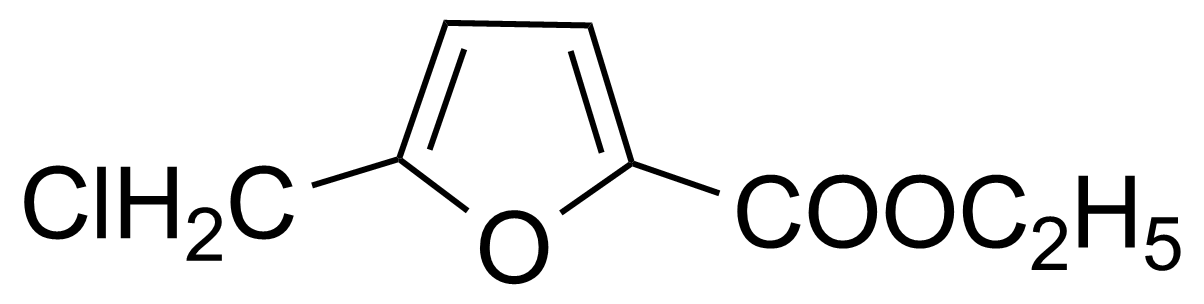 | [2528-00-9] | GEO-01329 |
| Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate | 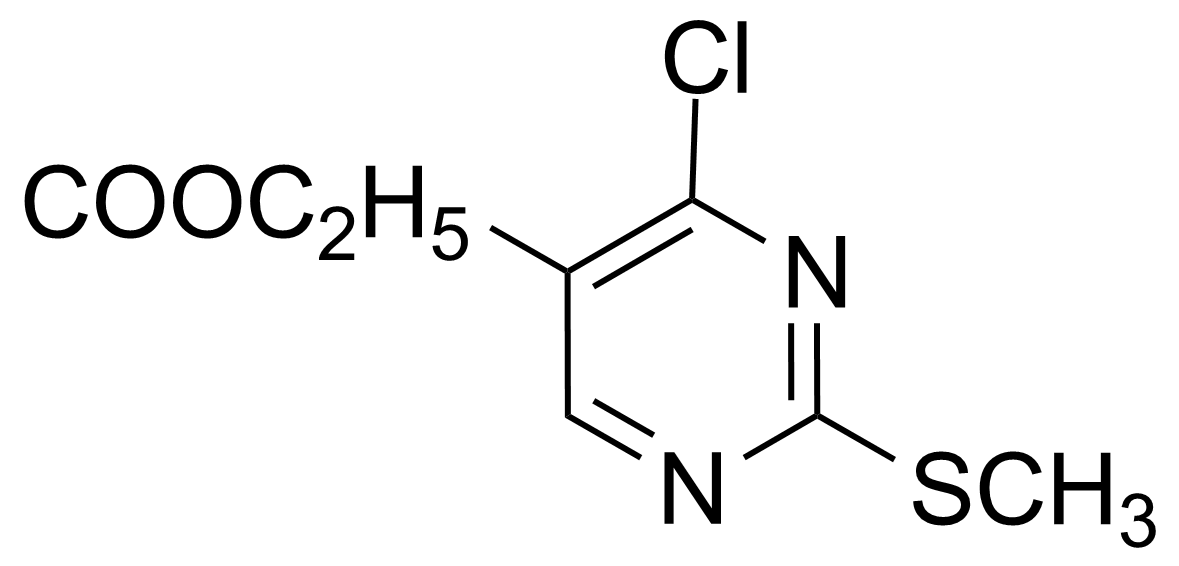 | [5909-24-0] | GEO-01330 |
| Ethyl 2-Chloro-4-trifluoromethyloxazole-5-carboxylate | 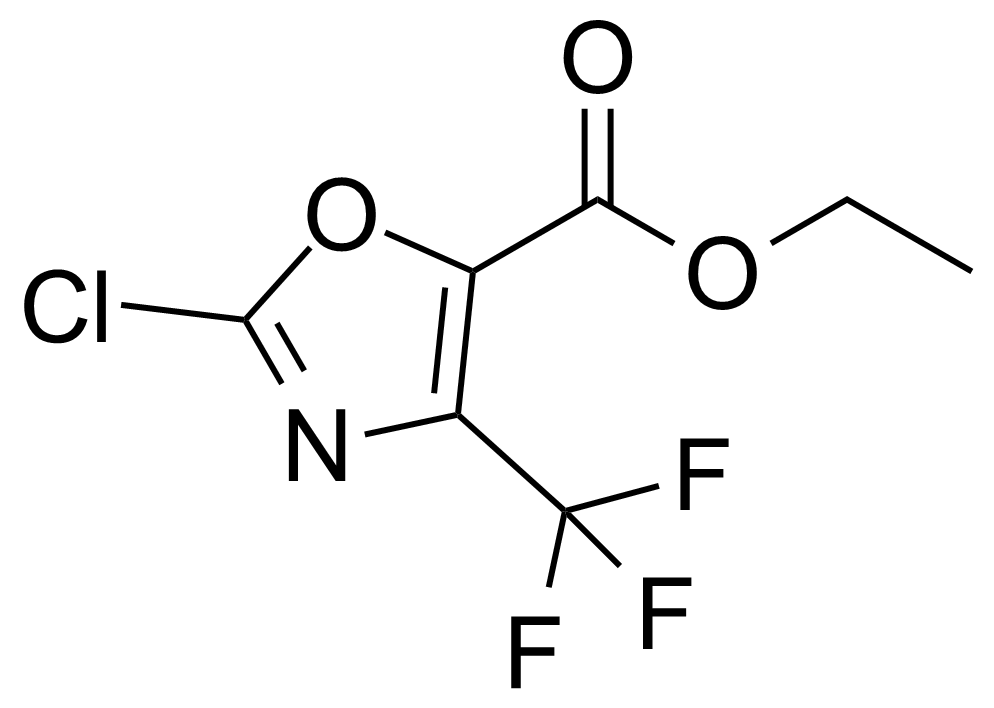 | [78451-14-6] | GEO-01332 |
| (E)-Ethyl 2-cyano-3-(1H-imidazol-4-yl)acrylate | 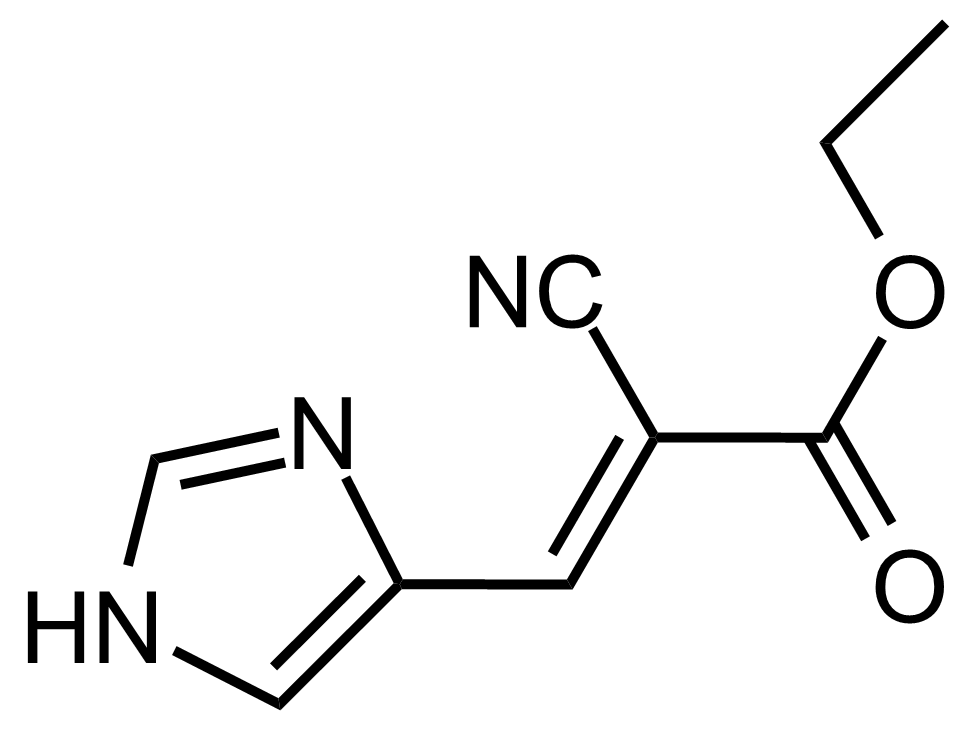 | [] | GEO-03647 |
| (Z)-Ethyl 2-cyano-3-(2-methyl-1H-indol-7-yl)acrylate | 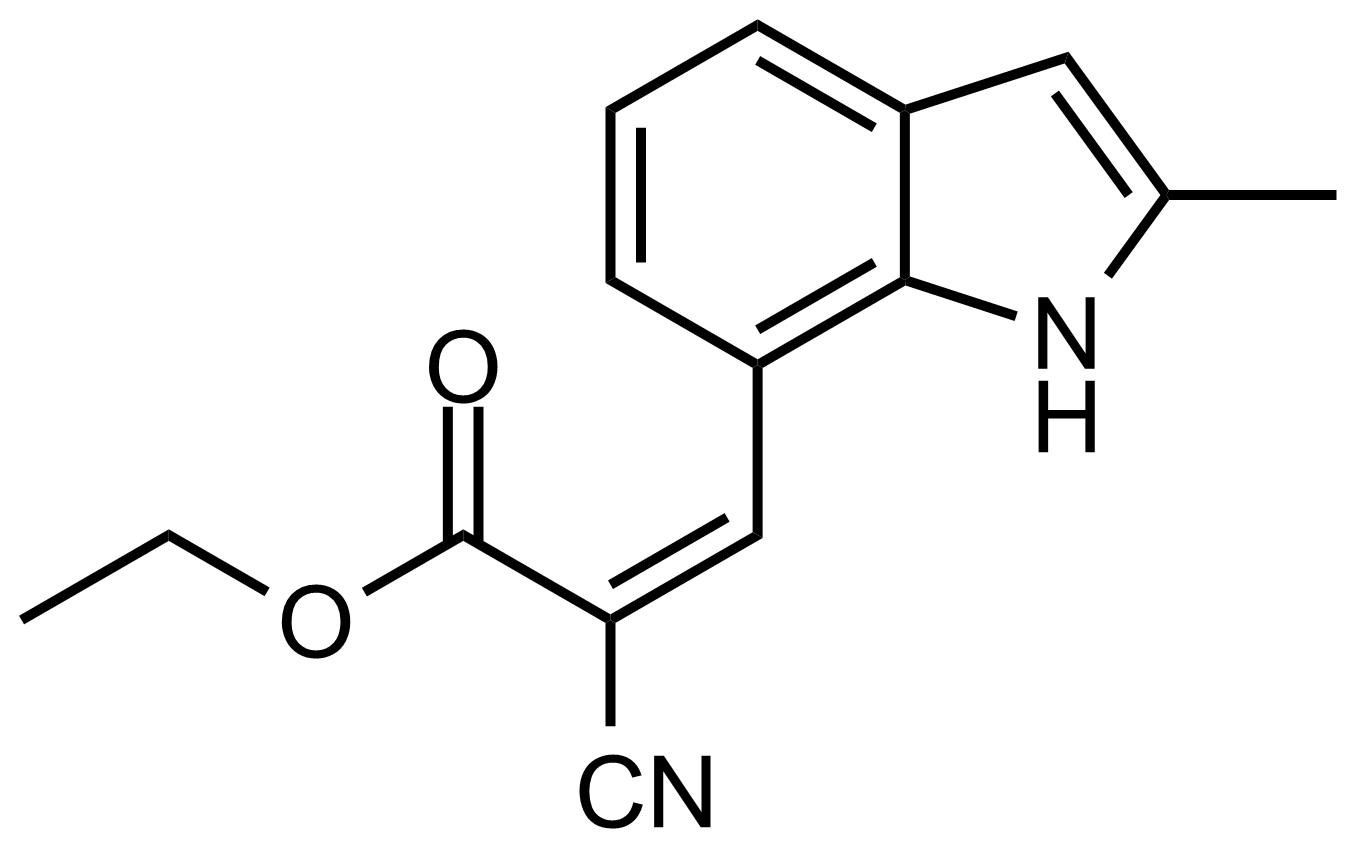 | [] | GEO-03636 |
| Ethyl ethoxyiminoacetate | 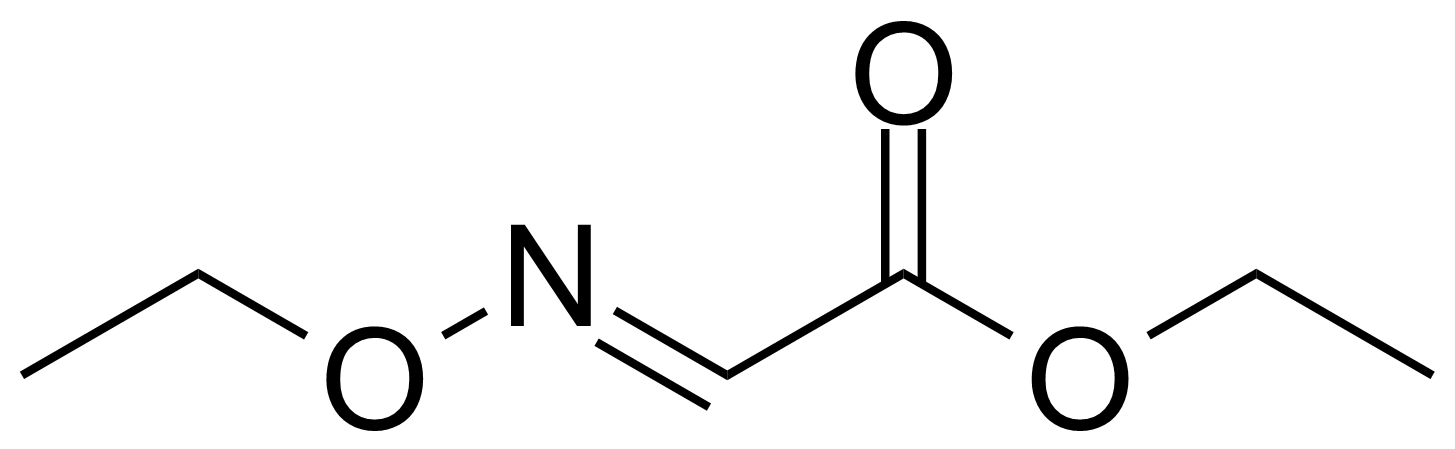 | [816-27-3] | GEO-01347 |
| Ethyl 2-ethyl-4,4,4-trifluoro-3-oxo-butanoate |  | [3854-50-0] | GEO-04050 |
| Ethyl 2-fluoro-3-oxo-3-phenylpropanoate | 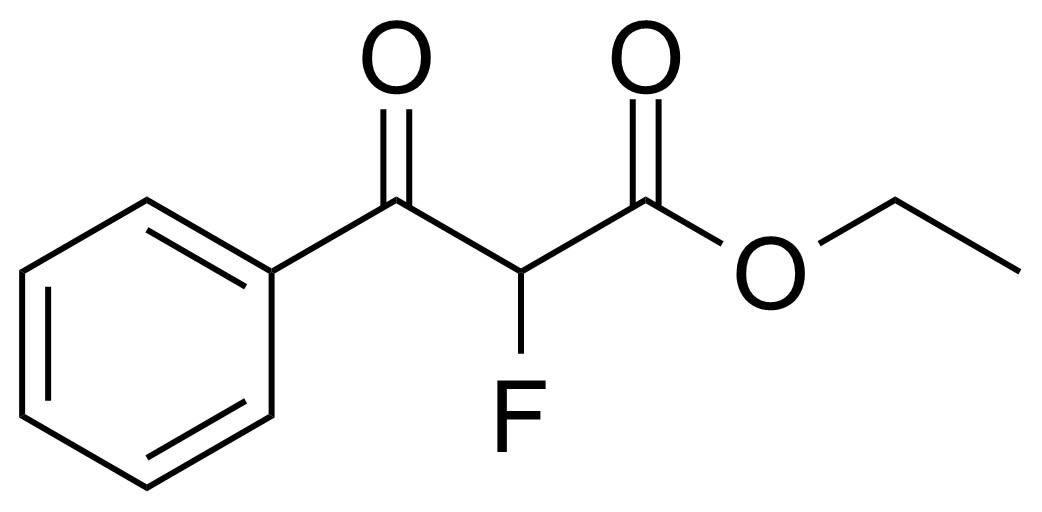 | [1479-22-7] | GEO-03233 |
| Ethyl 4-(3-fluorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 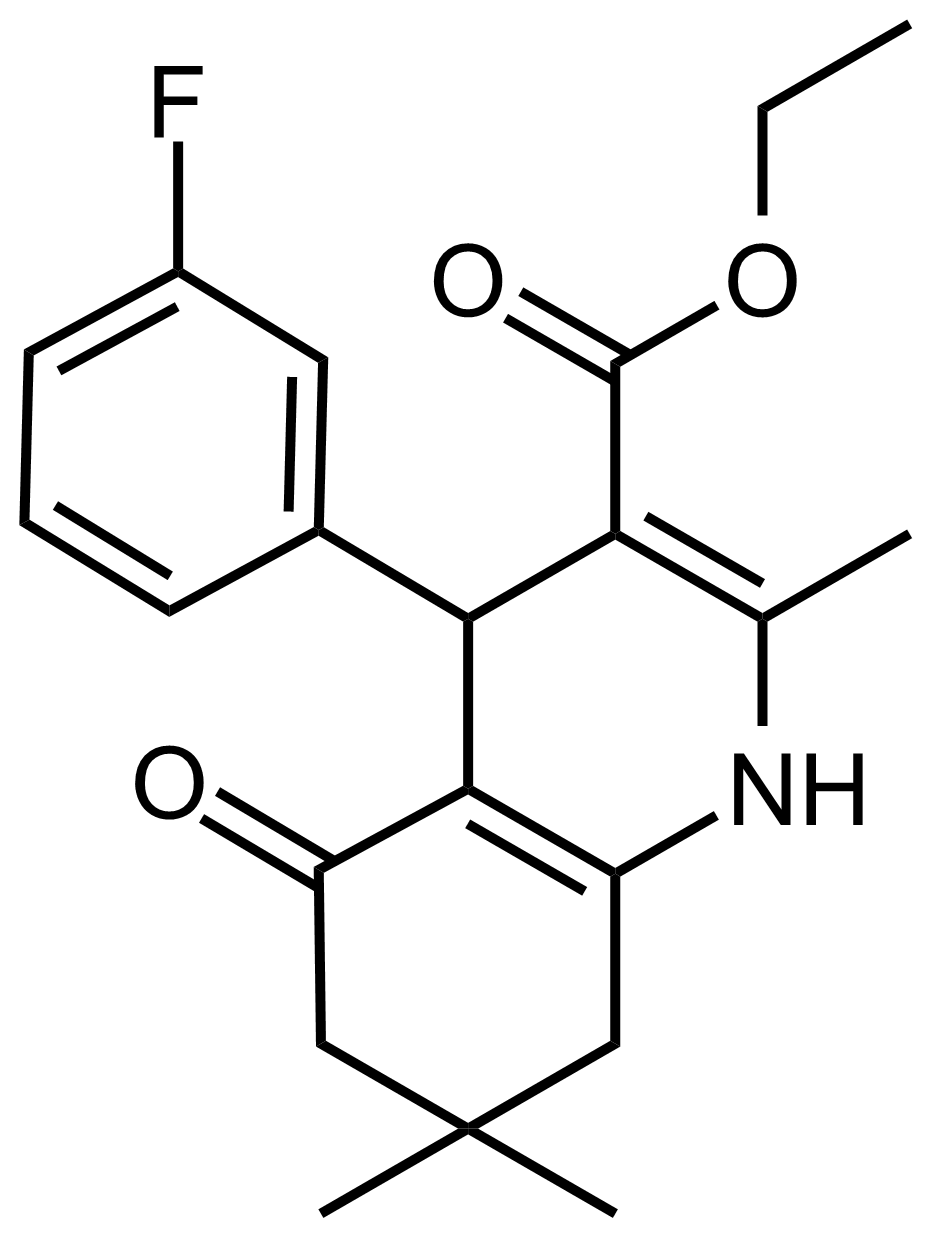 | N/A | GEO-03541 |
| Ethyl 4-(4-fluorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate |  | N/A | GEO-03559 |
| Ethyl 3-furoate | 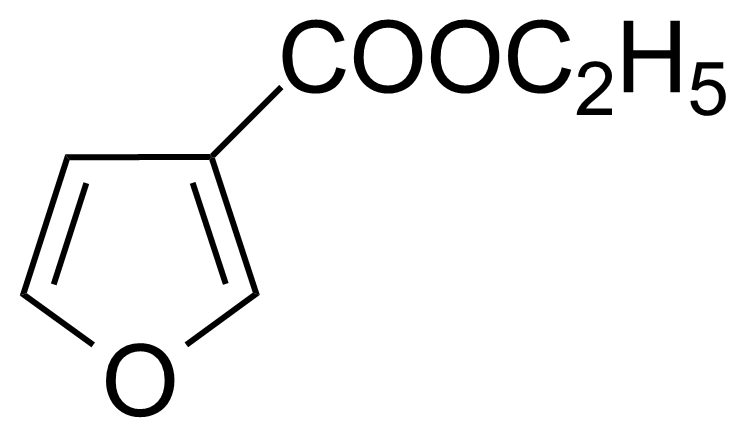 | [614-98-2] | GEO-03433 |
| Ethyl 2-isocyanatoacetate |  | [2949-22-6] | GEO-03266 |
| Ethyl 2-isocyanatobenzoate |  | [76393-16-3] | GEO-03779 |
| Ethyl 2-isocyanatopropionate | 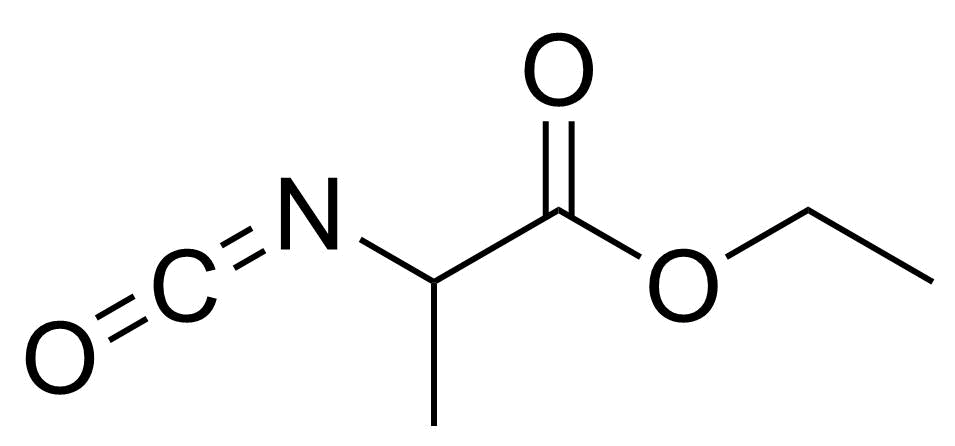 | [13794-28-0] | GEO-03007 |
| Ethyl 3-isocyanatopropionate | 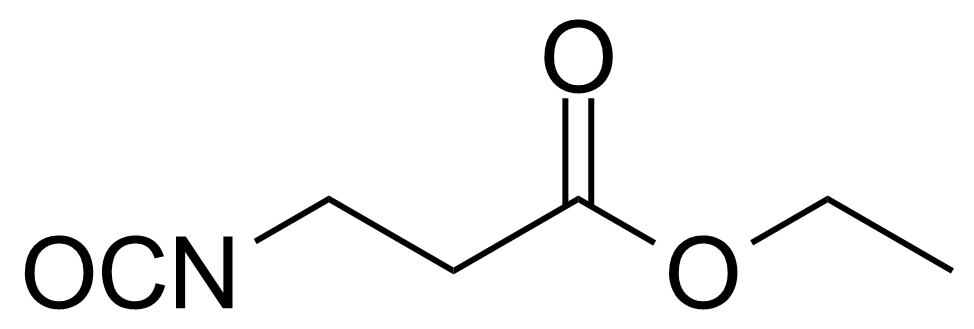 | [5100-34-5] | GEO-01357 |
| Ethyl isocyanoacetate | 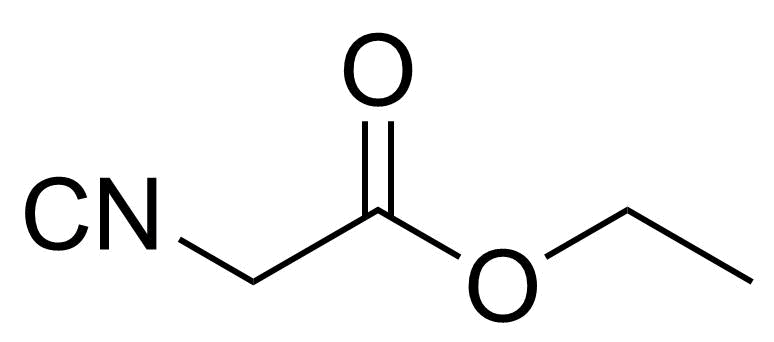 | [2999-46-4] | GEO-01356 |
| Ethyl 3-methyl-4H-1,4-benzothiazine-2-carboxylate |  | [7625-01-6] | GEO-01359 |
| Ethyl 2-methylindole-3-carboxylate |  | [53855-47-3] | GEO-03725 |
| New | Ethyl 5-(1-methyl-2-nitro-1H-imidazole-5-carboxamido)-1-methyl-1H-imidazole-2-carboxylate | 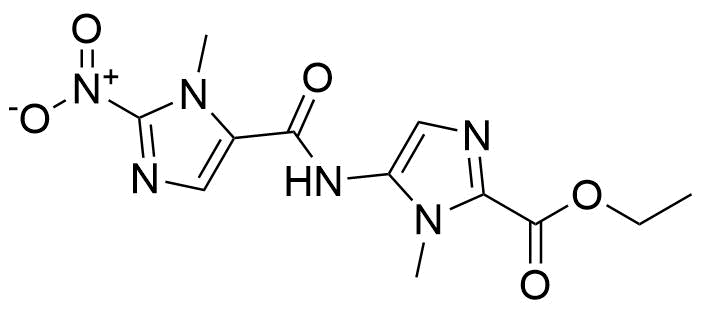 | [N/A] | GEO-01362 |
| Ethyl 5-nitrobenzo[b]furan-2-carboxylate | 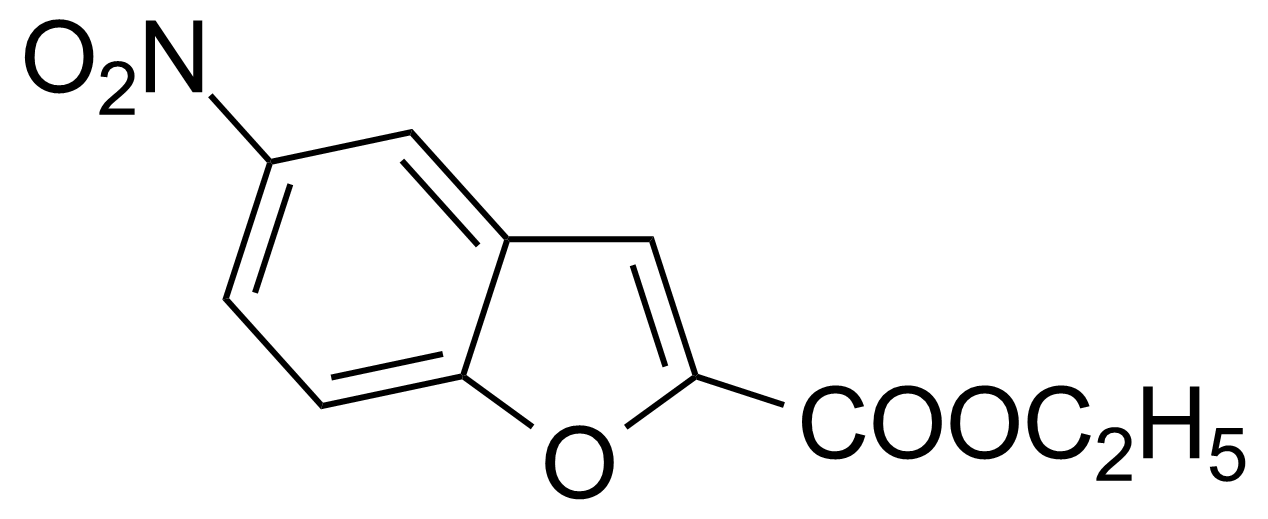 | [69604-00-8] | GEO-01364 |
| Ethyl 5-nitro-2-furoate | 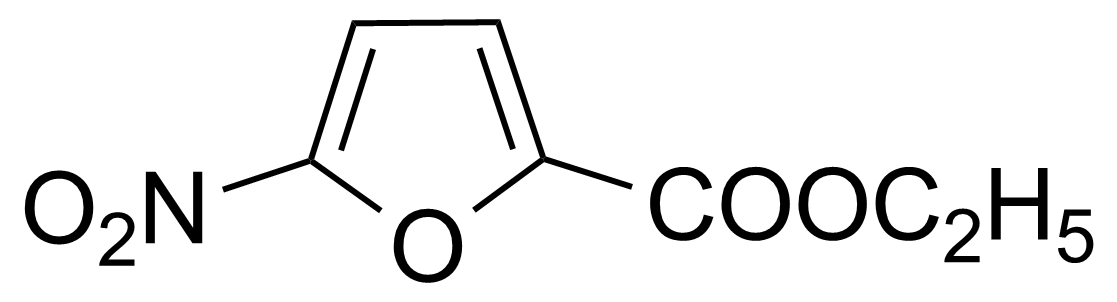 | [943-37-3] | GEO-01365 |
| Ethyl phenylcyanoacetate | 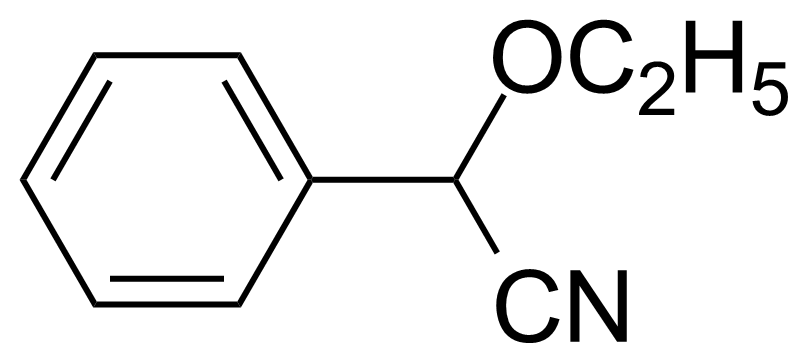 | [4553-07-5] | GEO-01372 |
| Ethyl 1H-pyrazole-3-carboxylate | 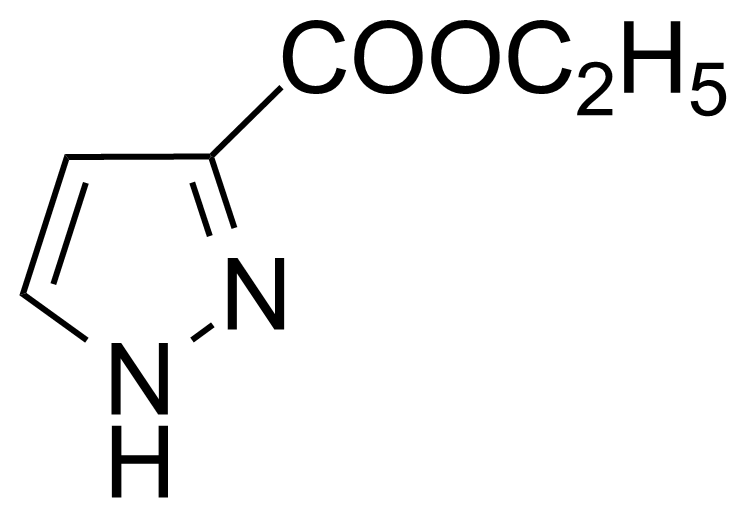 | [5932-27-4] | GEO-01374 |
| Ethyl (S)-(+)-2-pyrrolidone-5-carboxylate | 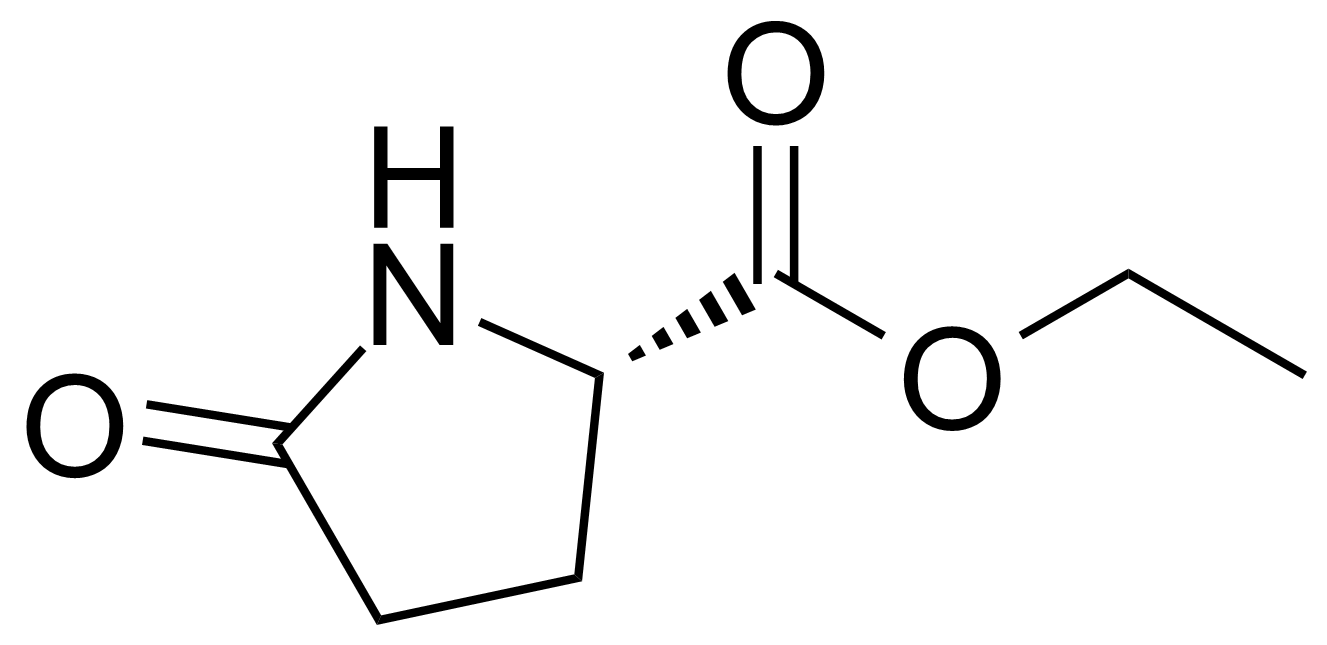 | [7149-65-7] | GEO-01377 |
| Ethyl 3-thiophenecarboxylate | 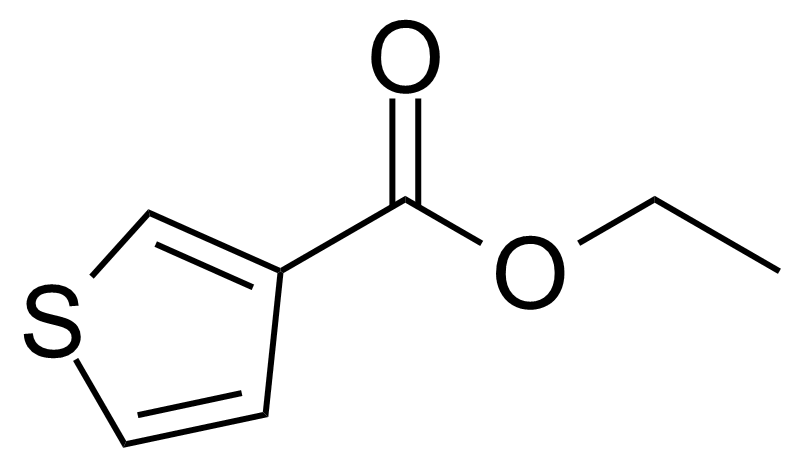 | [5751-80-4] | GEO-03865 |
| Ethyl 2,7,7-trimethyl-4-(3-methylpyridin-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 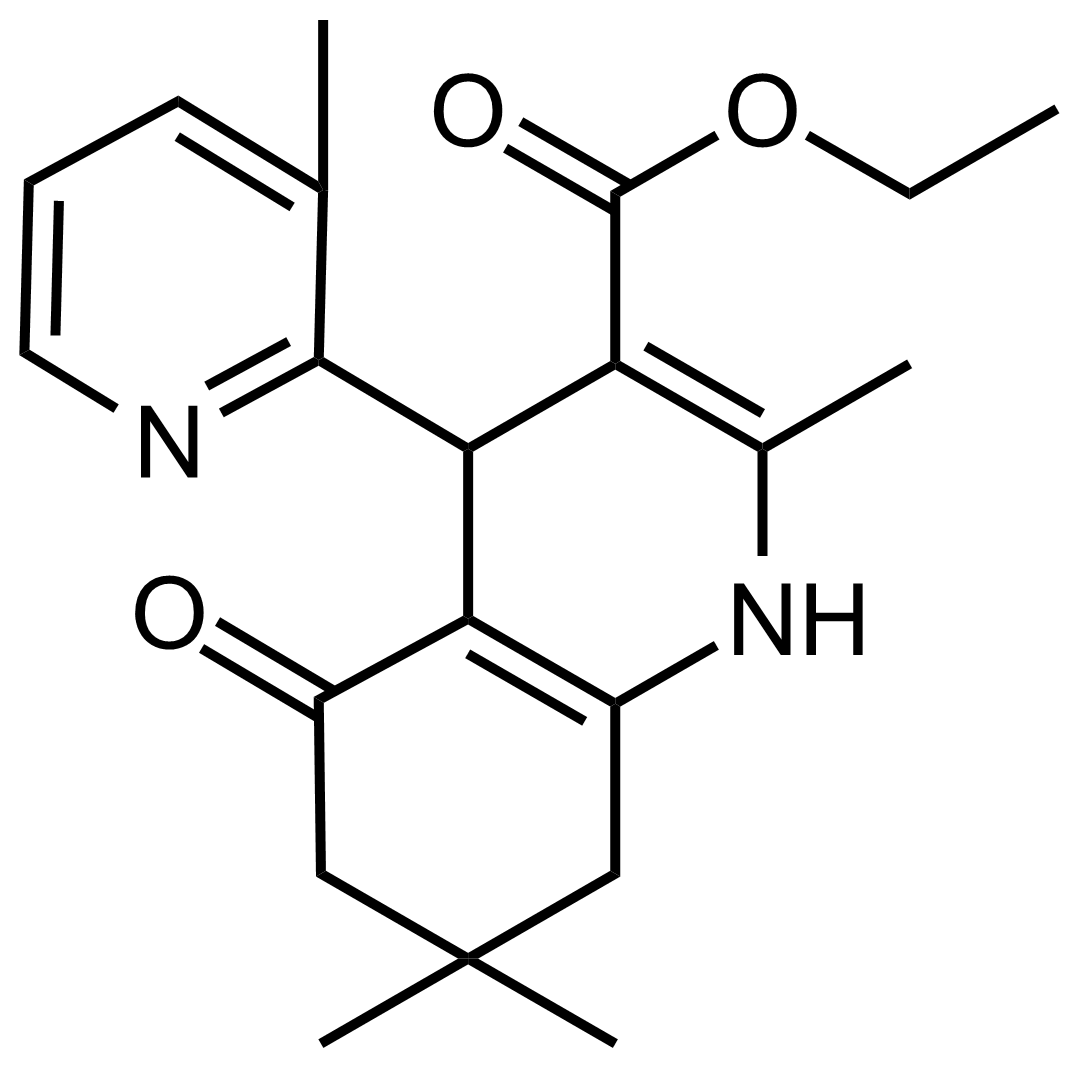 | N/A | GEO-03537 |
| Ethyl 2,7,7-trimethyl-4-(5-((5-methyl-1,3,4-thiadiazol-2-yl)thio)furan-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 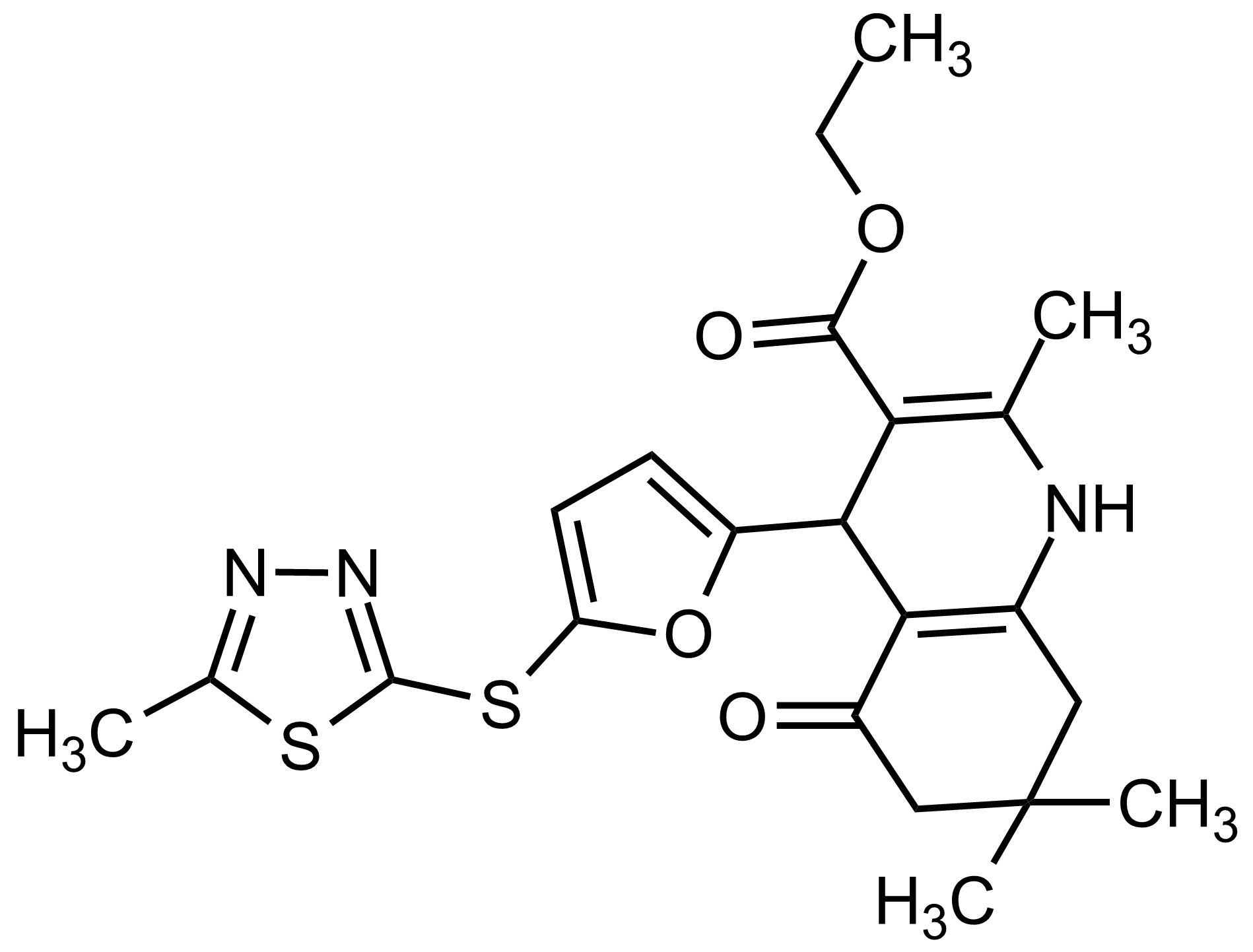 | N/A | GEO-03545 |
| (S)-(+)-gamma-Hydroxymethyl-gamma-butyrolactone | 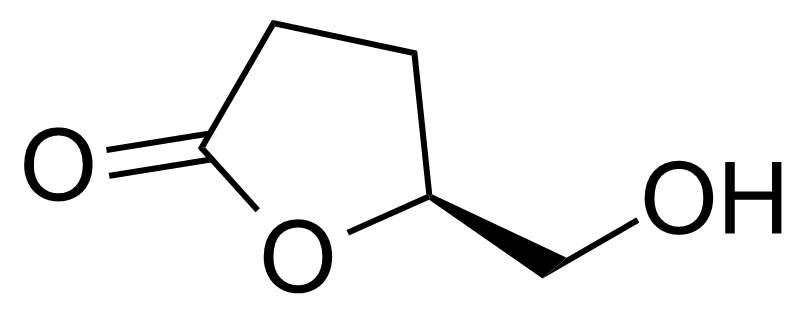 | [32780-06-6] | GEO-04229 |
| 4-Hydroxy-2(5H)-furanone | 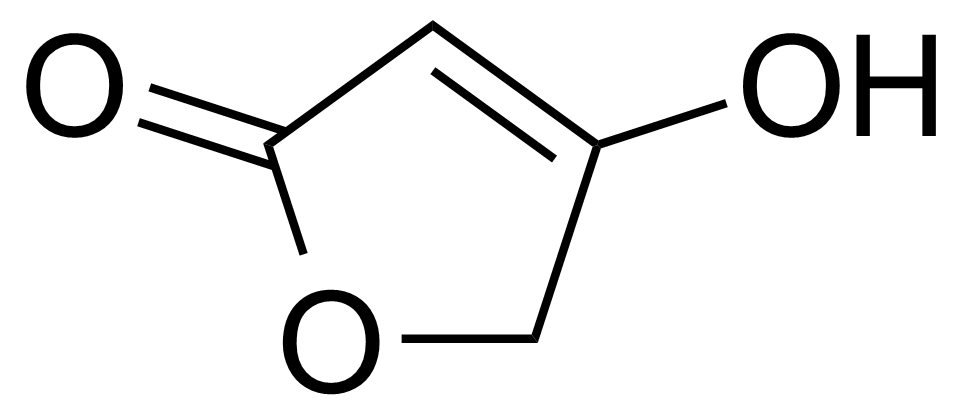 | [541-57-1] | GEO-01517 |
| 5-Hydroxy-4-methyl-2(5H)-furanone | 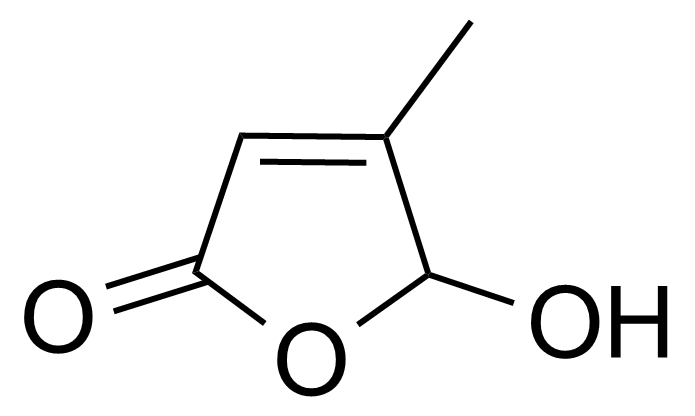 | [40834-42-2] | GEO-03047 |
| (2S)-2-(Hydroxymethyl)-2H-furan-5-one | 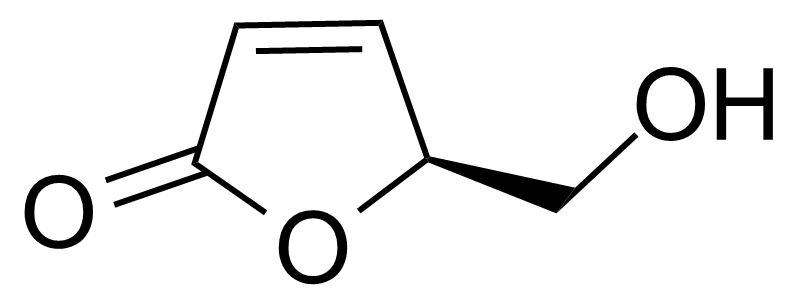 | [78508-96-0] | GEO-03017 |
| 5-Hydroxypyrazine-2-carboxylic acid methyl ester | 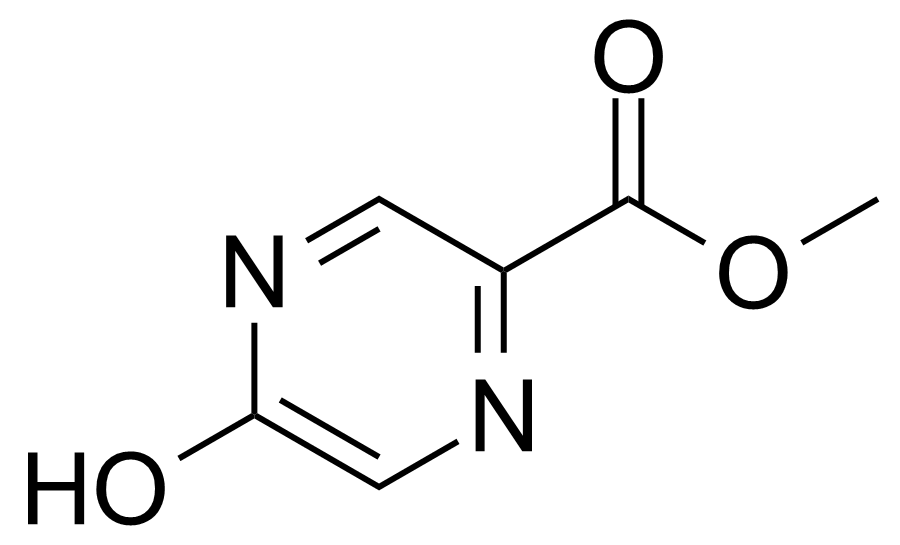 | [13924-95-3] | GEO-01557 |
| New | 4-Hydroxy-TEMPO-benzoate | 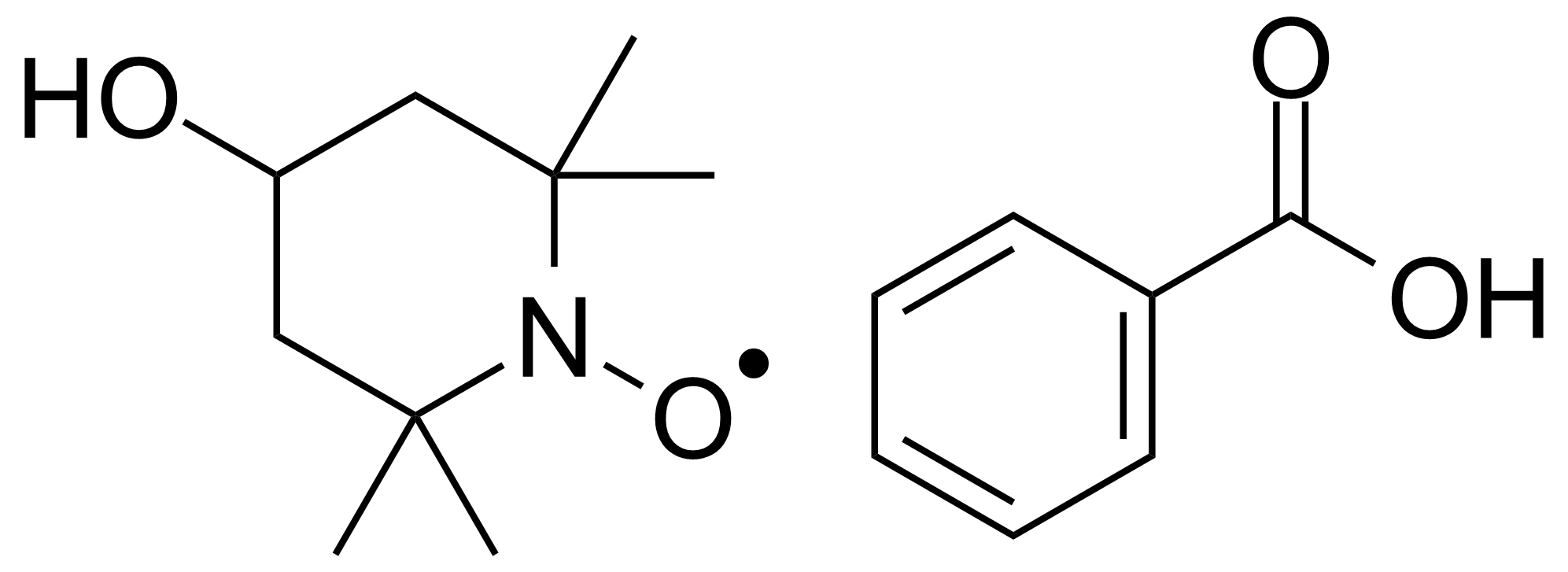 | [3225-26-1] | GEO-01563 |
| 4-Iodobutyl acetate (tech.) | 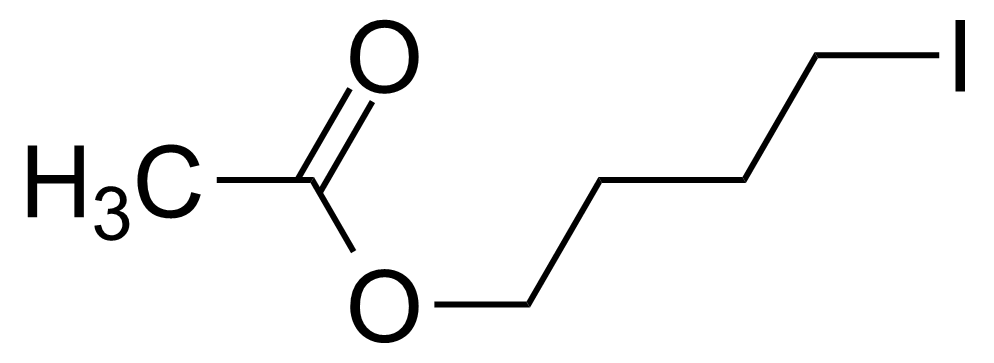 | [40596-44-9] | GEO-01596 |
| New | Isoamyl-3-(2-furyl)propionate | 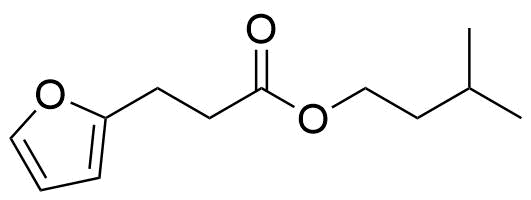 | [7779-67-1] | GEO-01608 |
| 3-Isothiocyanato-2-methylbenzoic acid methyl ester | 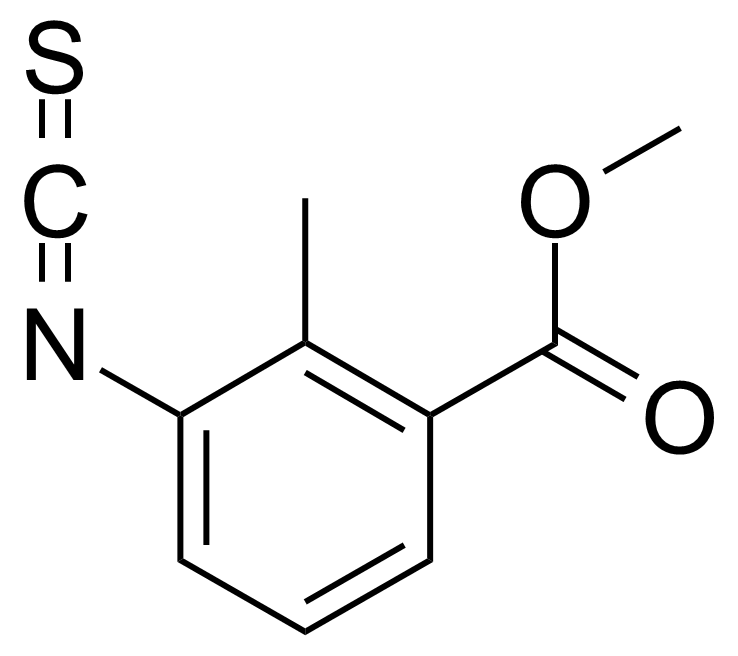 | [1001185-62-1] | GEO-02715 |
| 4-Methoxycarbonylphenyl chloroformate | 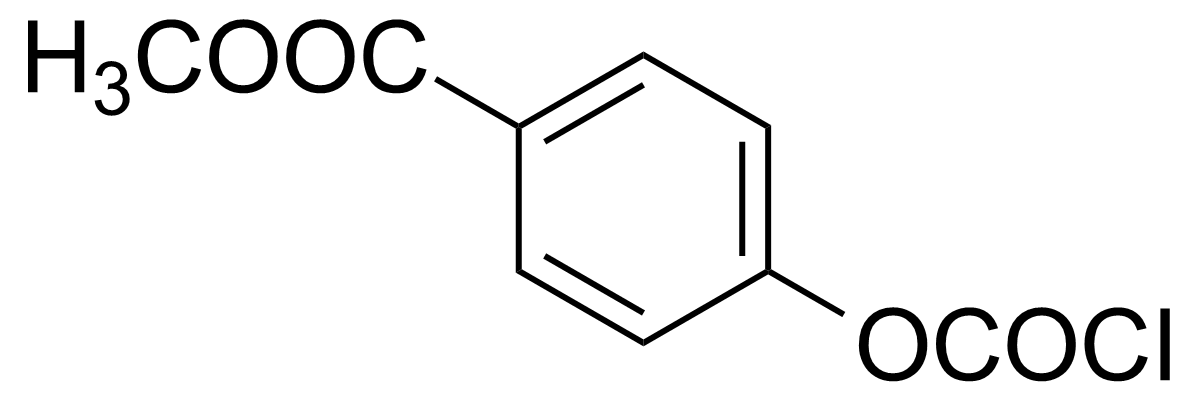 | [31140-40-6] | GEO-01675 |
| 3-(Methoxycarbonyl)phenyl isothiocyanate | 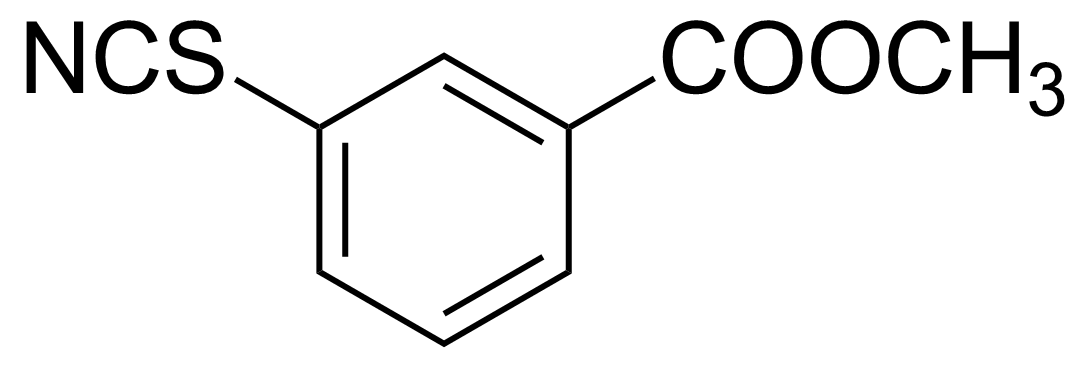 | [3125-66-4] | GEO-03396 |
| 4-Methoxycarbonylphenyl isothiocyanate | 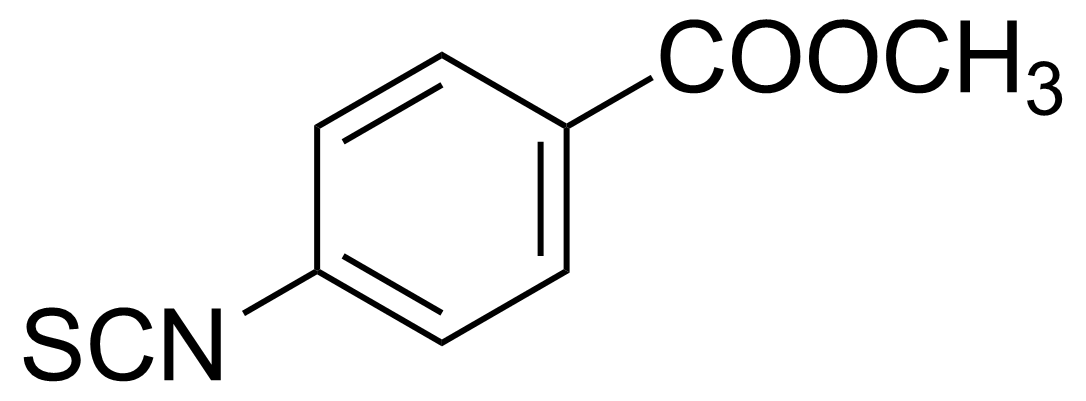 | [3662-78-0] | GEO-01676 |
| 4-Methoxy-2(5H)-furanone | 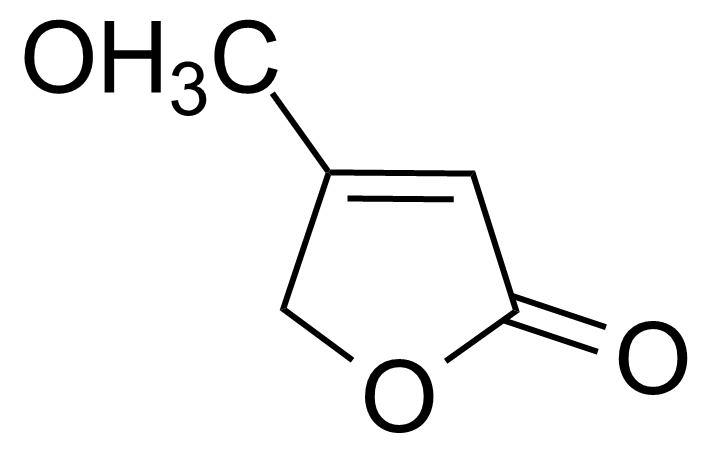 | [69556-70-3] | GEO-01680 |
| 4-(Methylamino)benzoic acid ethyl ester | 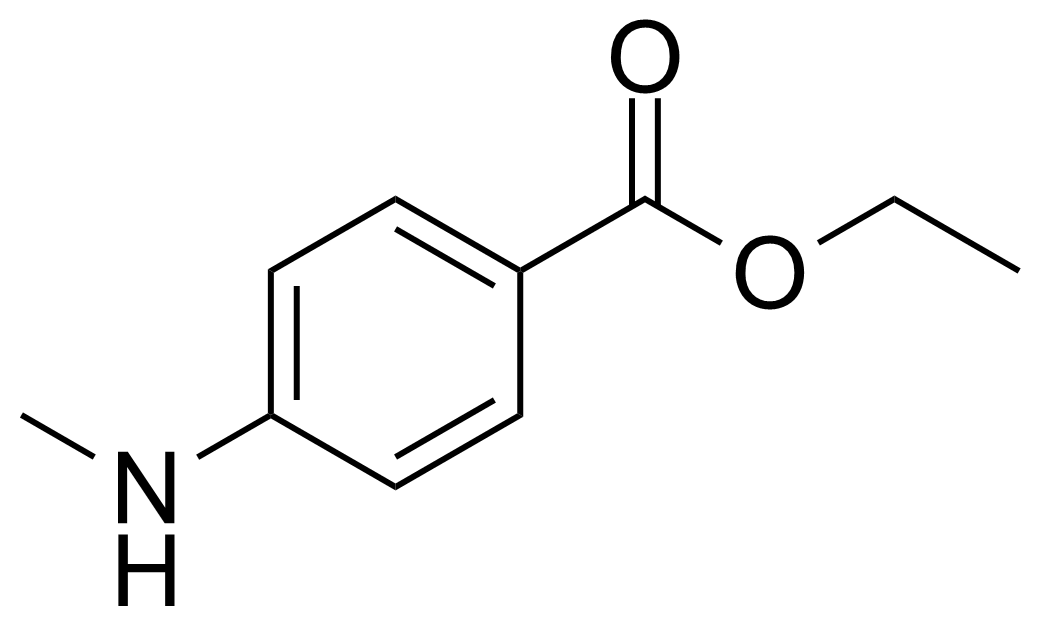 | [10541-82-9] | GEO-04018 |
| Methyl 3-amino-4-methylthiophene-2-carboxylate | 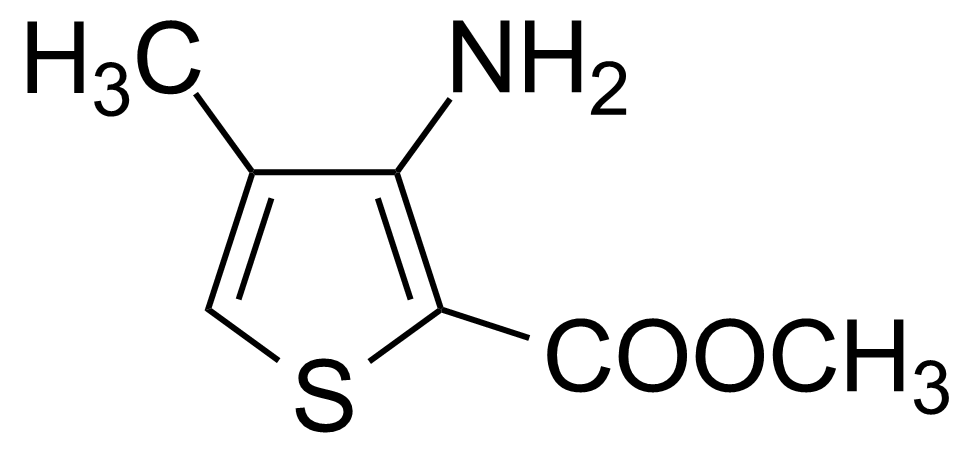 | [85006-31-1] | GEO-01745 |
| Methyl 6-aminonicotinate | 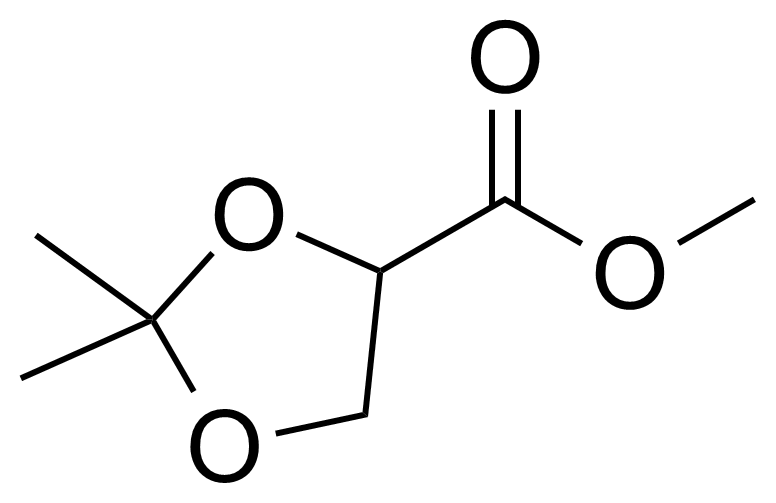 | [36052-24-1] | GEO-00186 |
| Methyl 3-amino-2-thiophenecarboxylate |  | [22288-78-4] | GEO-01748 |
| Methyl 2-amino-3-thiophenecarboxylate | 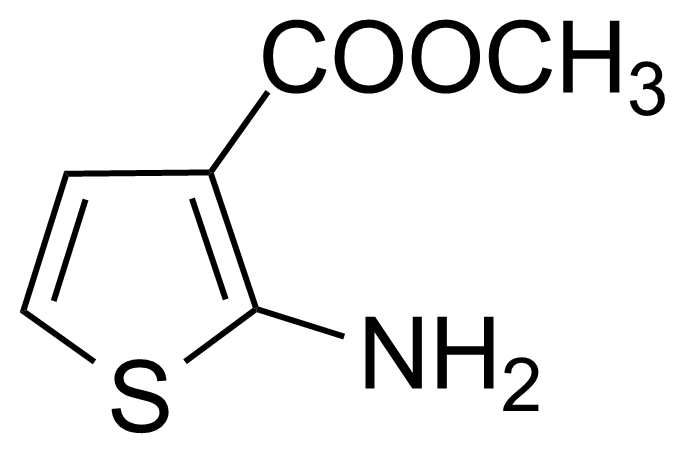 | [4651-81-4] | GEO-01747 |
| 3-Methylbenzofuran-2-carboxylic acid ethyl ester | 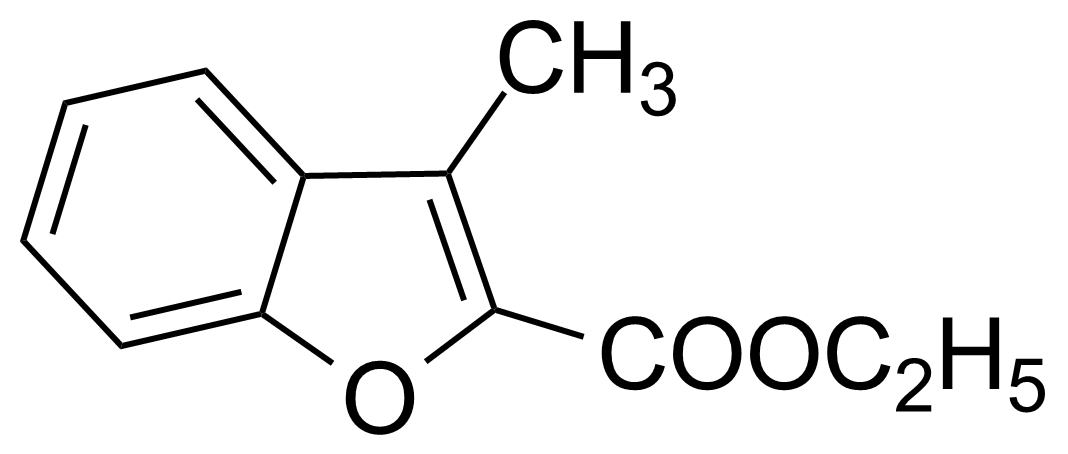 | [22367-82-4] | GEO-01765 |
| (E)-Methyl 3-(5-bromo-2-fluorophenyl)-2-cyanoacrylate | 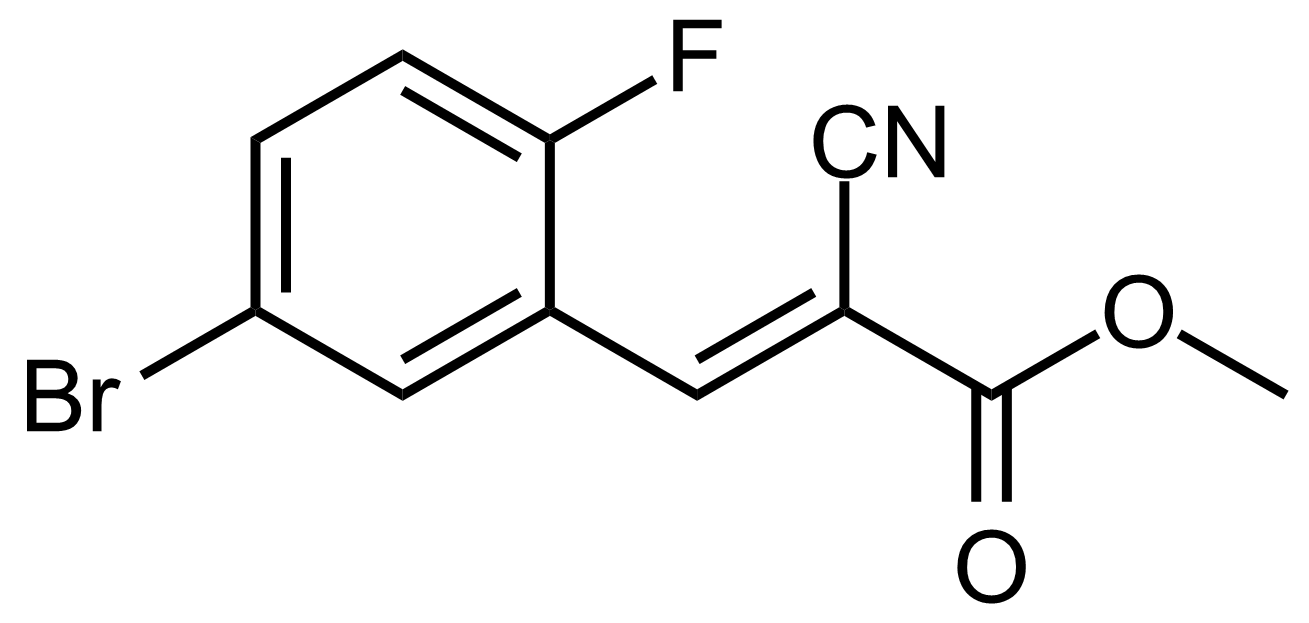 | [] | GEO-03626 |
| Methyl 5-bromo-2-furancarboxylate | 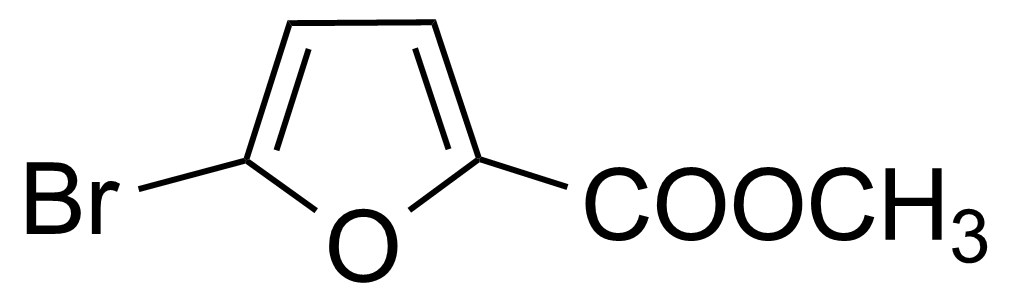 | [2527-99-3] | GEO-03018 |
| Methyl 2-bromo-4-nitrobenzoate | 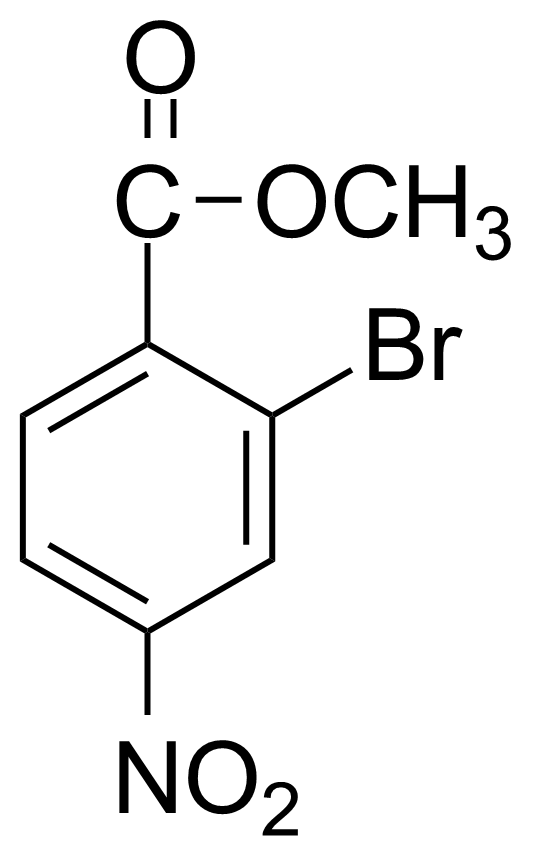 | [100959-22-6] | GEO-03933 |
| (E)-Methyl 3-(3-bromopyridin-4-yl)-2-cyanoacrylate | 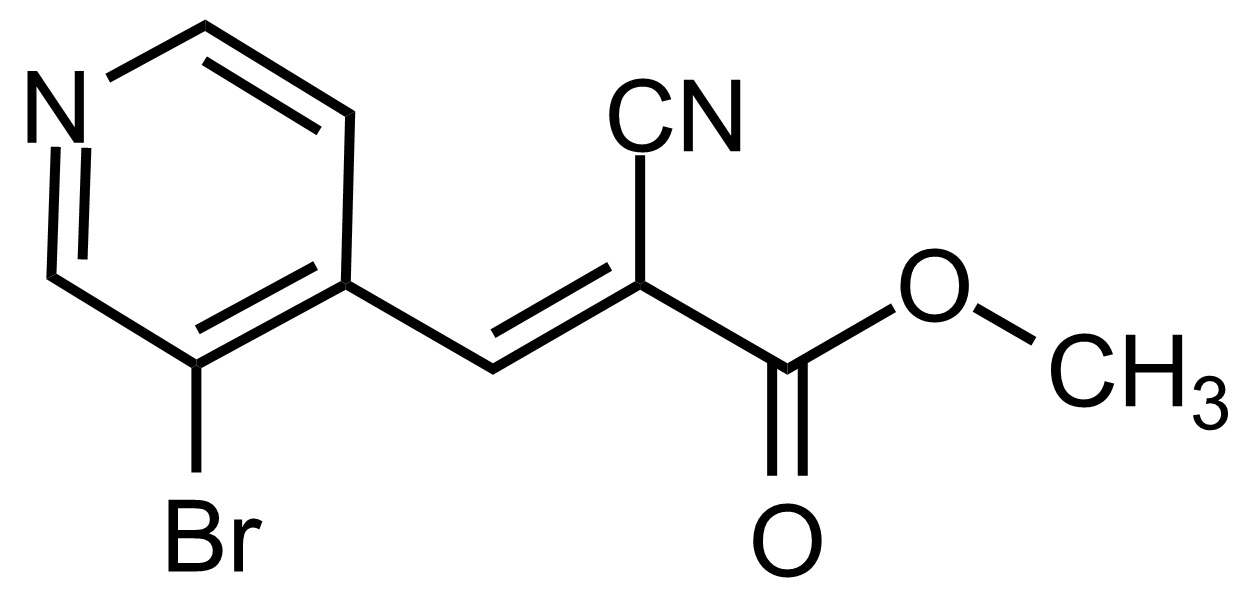 | N/A | GEO-03565 |
| (E)-Methyl 3-(6-bromopyridin-2-yl)-2-cyanoacrylate | 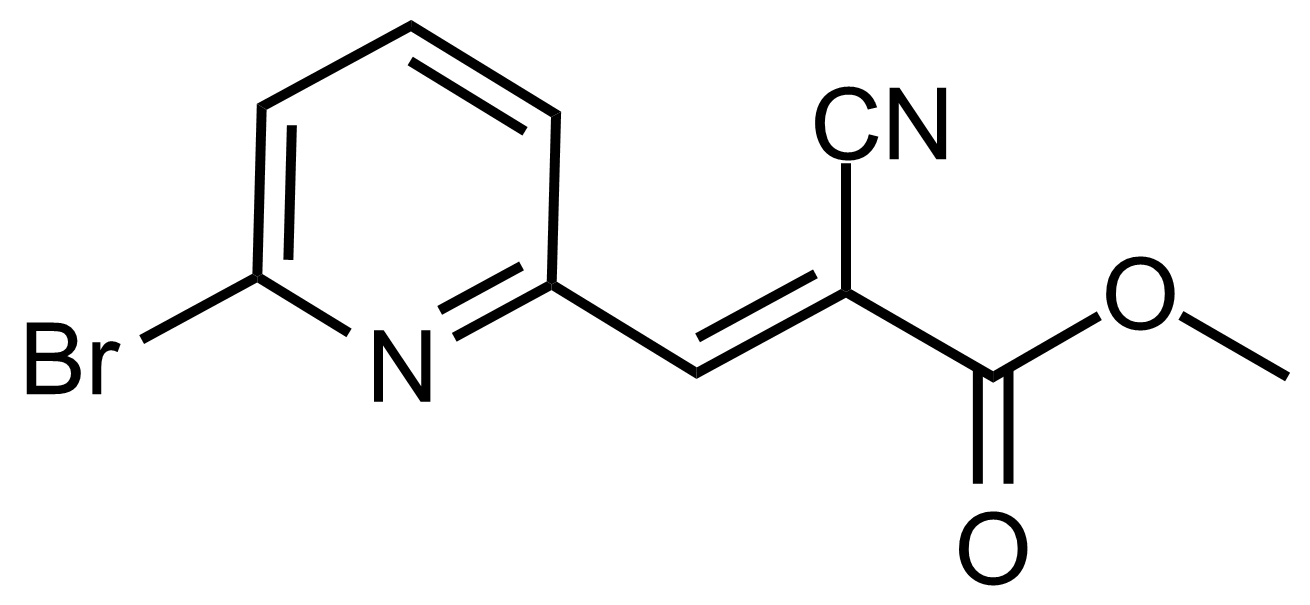 | N/A | GEO-03512 |
| Methyl 5-bromosalicylate | 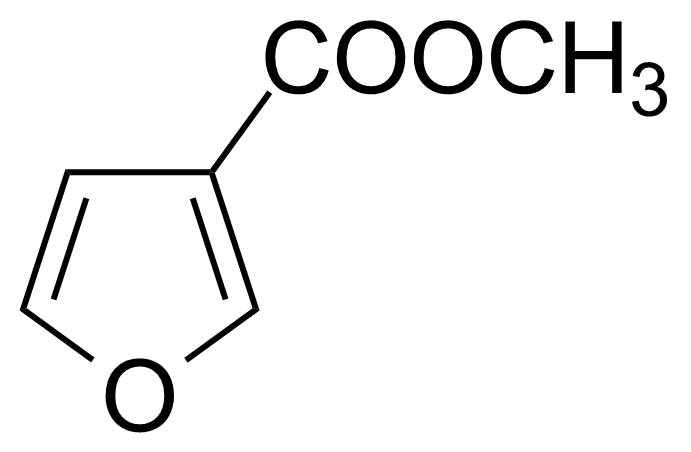 | [4068-76-2] | GEO-01789 |
| Methyl 2-bromothiazole-5-carboxylate | 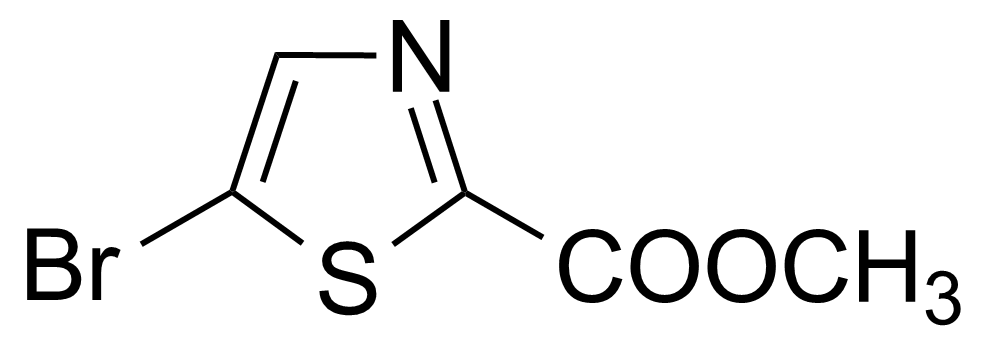 | [54045-74-8] | GEO-01790 |
| (E)-Methyl 3-(4-bromothiophen-3-yl)-2-cyanoacrylate | 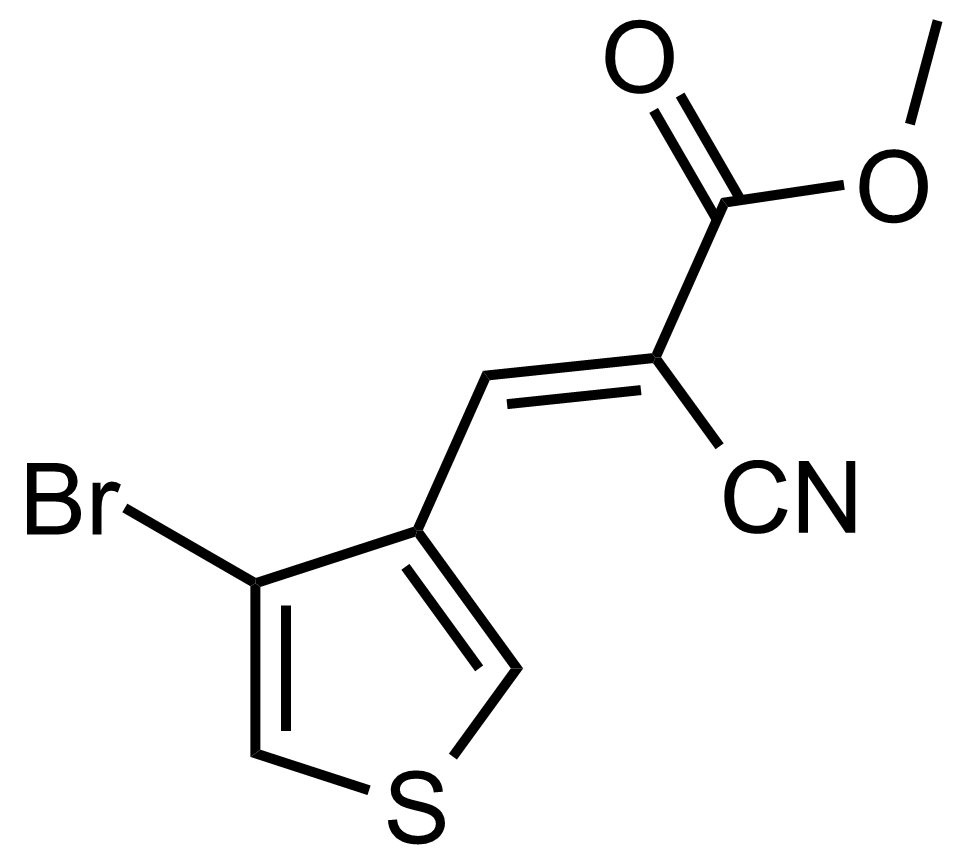 | [] | GEO-03661 |
| Methyl 4-chloroacetoacetate |  | [32807-28-6] | GEO-01793 |
| Methyl 2-Chloro-5-formylbenzoate | 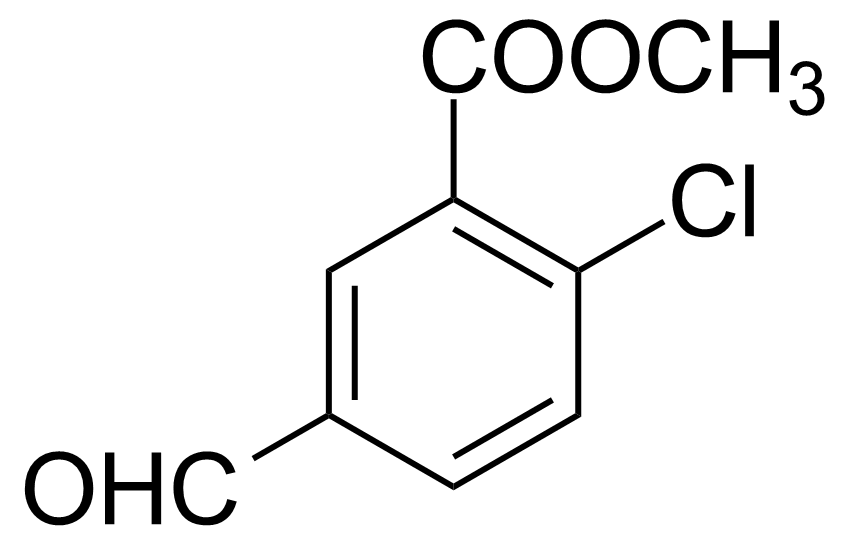 | [199679-23-7] | GEO-02577 |
| Methyl 4-(chloroformyl)butyrate |  | [1501-26-4] | GEO-01794 |
| (E)-Methyl 3-(2-chloro-4-iodopyridin-3-yl)-2-cyanoacrylate | 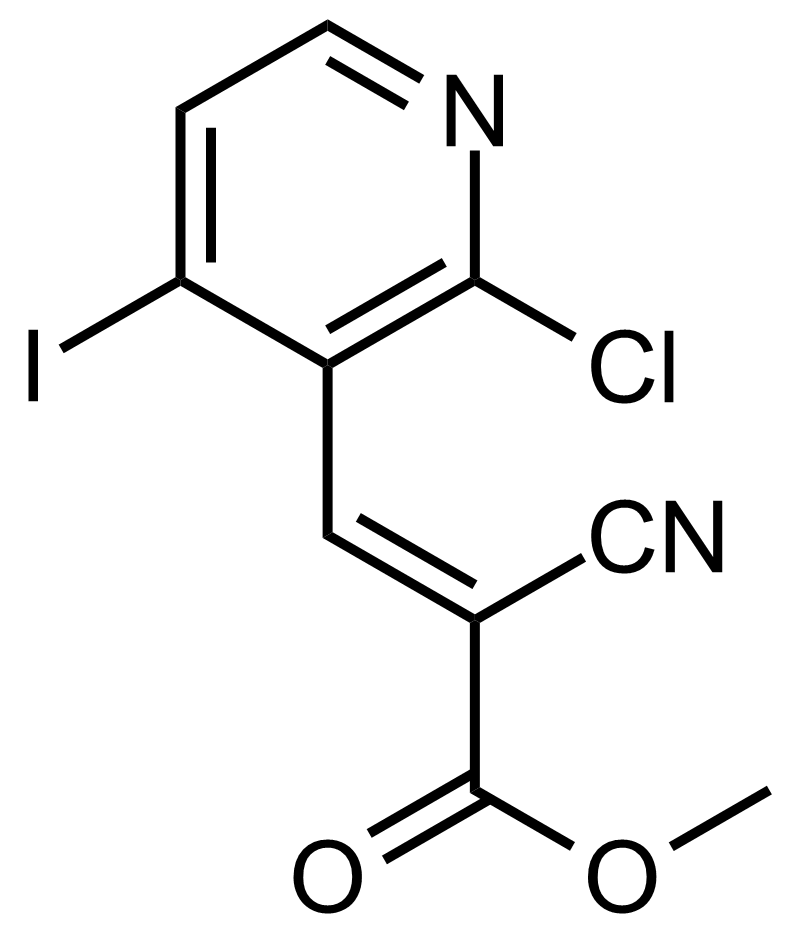 | [] | GEO-03668 |
| (E)-Methyl 3-(3-chloro-4-methoxyphenyl)-2-cyanoacrylate | 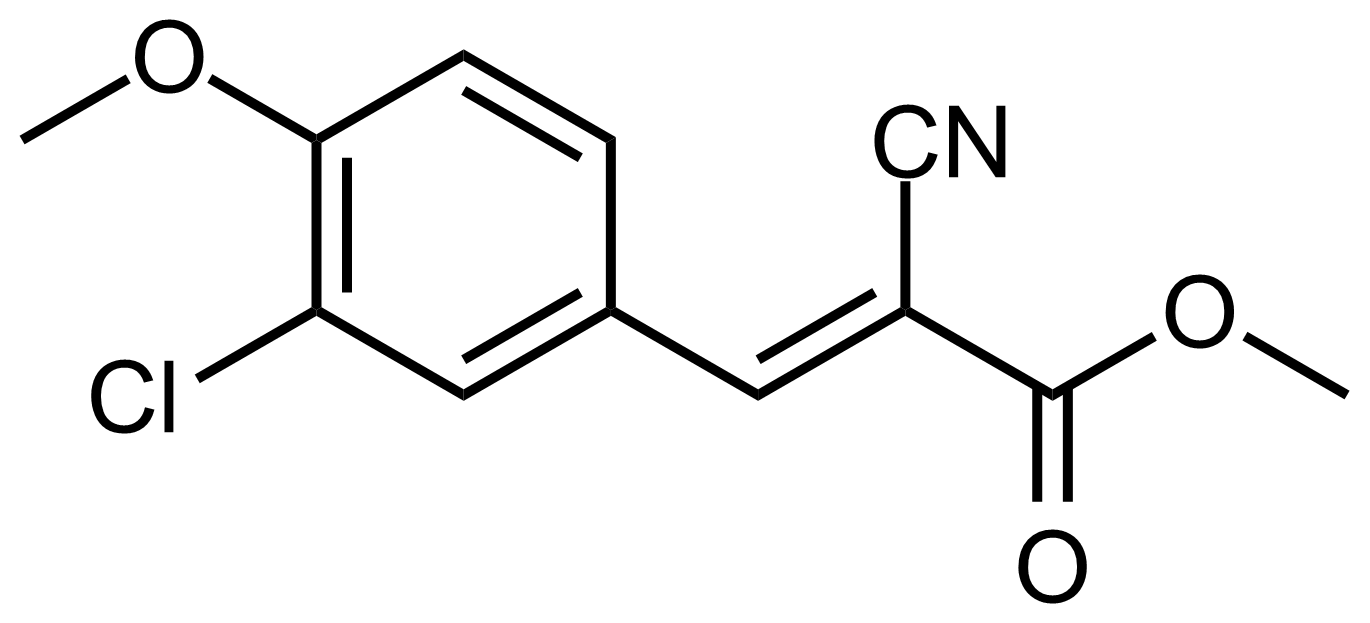 | N/A | GEO-03611 |
| (E)-Methyl 3-(5-(3-chloro-4-methoxyphenyl)furan-2-yl)-2-cyanoacrylate |  | [] | GEO-03643 |
| Methyl 4-(3-chloro-4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 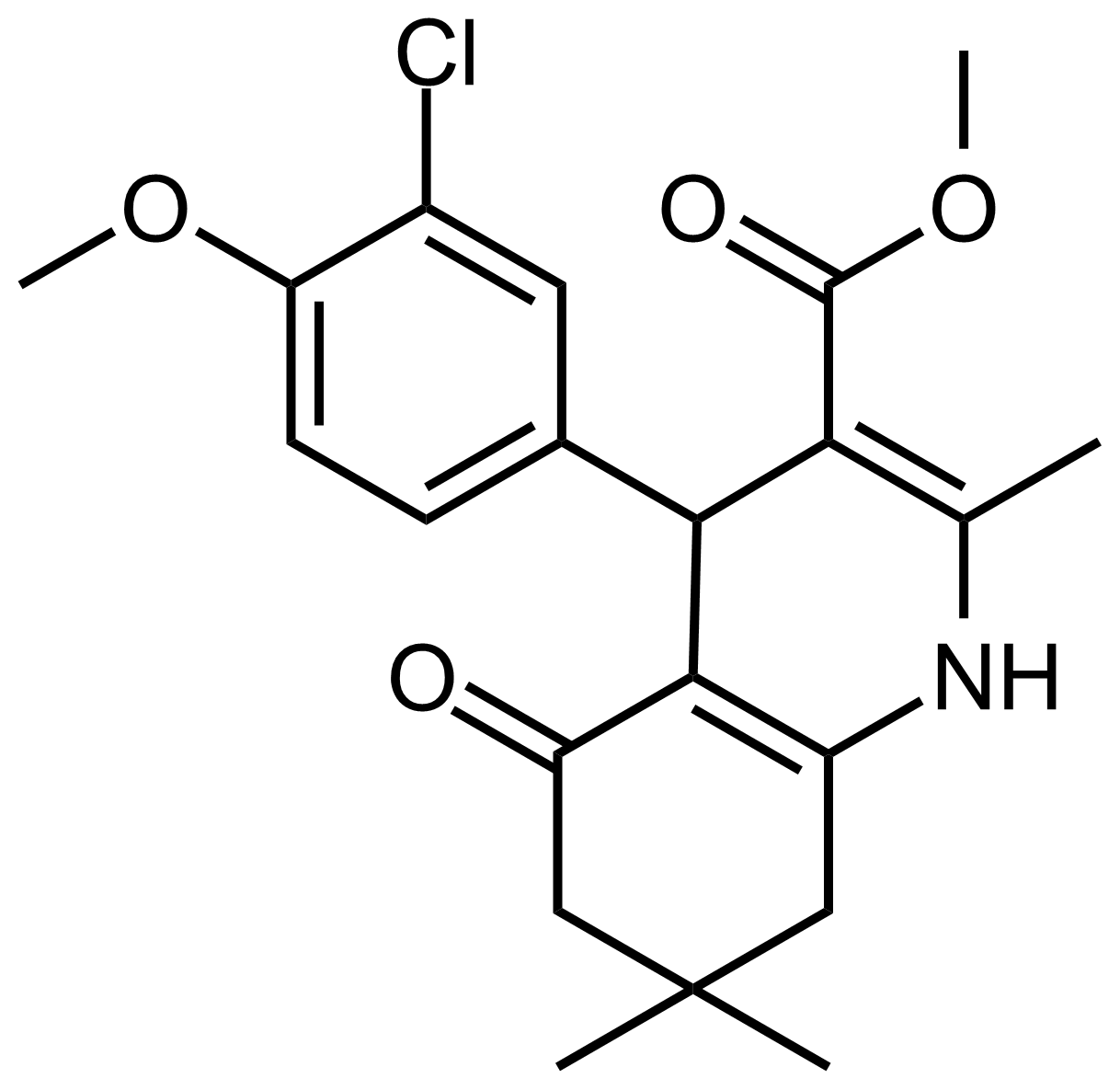 | [] | GEO-03615 |
| Methyl 5-(chloromethyl)-2-furoate | 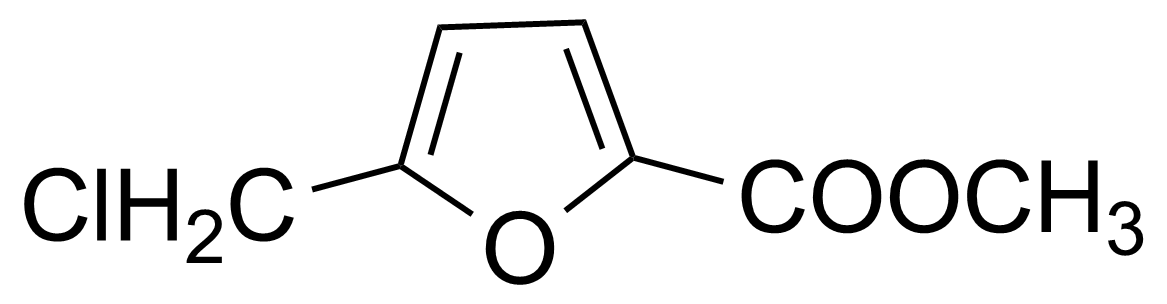 | [2144-37-8] | GEO-01797 |
| Methyl 4-[(chlorosulfonyl)methyl]benzoate | ![Structure of Methyl 4-[(chlorosulfonyl)methyl]benzoate](https://georganics.sk/wp-content/uploads/2021/05/GEO-03044_Methyl_4-chlorosulfonylmethylbenzoate.png) | [130047-14-2] | GEO-03044 |
| Methyl 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylate | 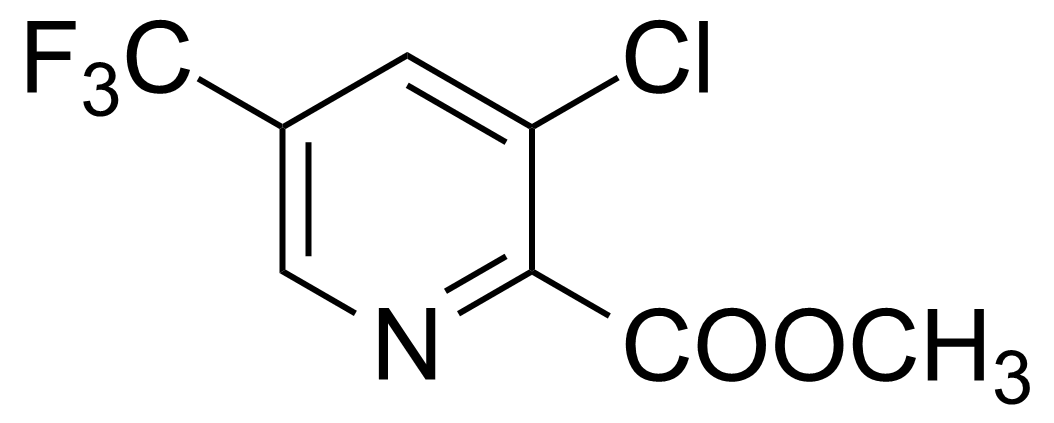 | [655235-65-7] | GEO-03392 |
| (E)-Methyl 2-cyano-3-(5-hexylthiophen-2-yl)acrylate | 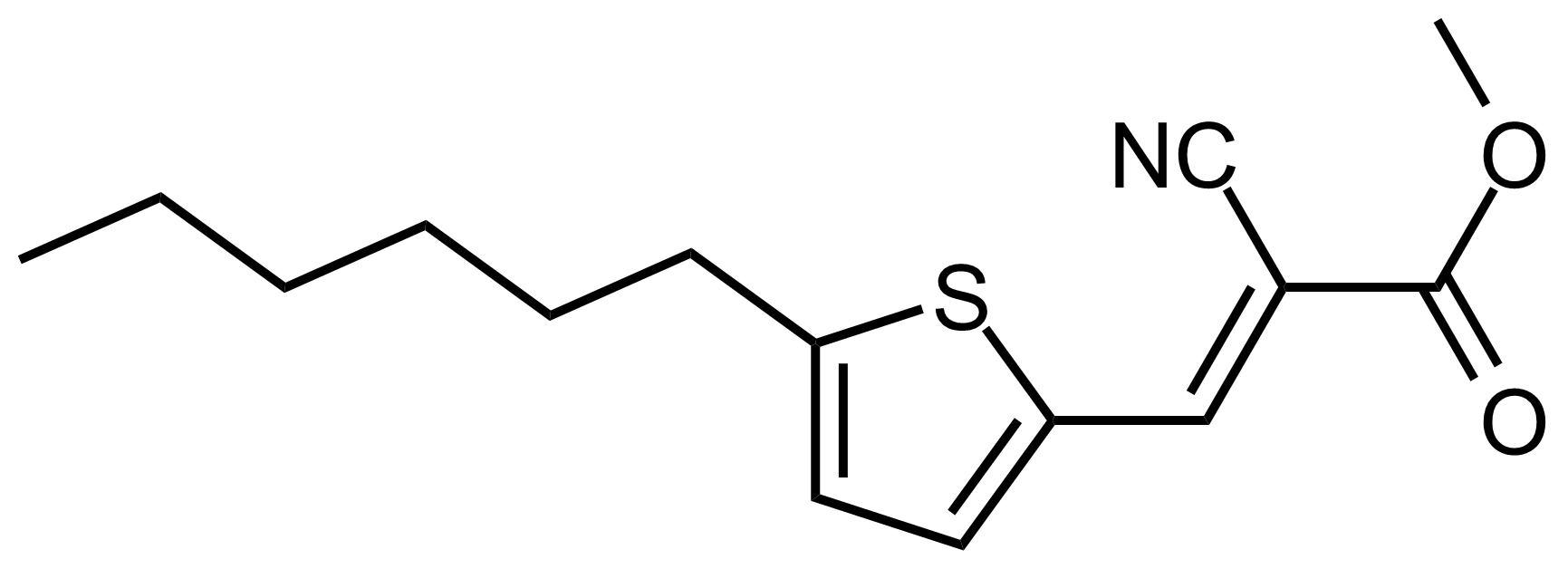 | N/A | GEO-03507 |
| (E)-Methyl 2-cyano-3-(1H-imidazol-4-yl)acrylate | 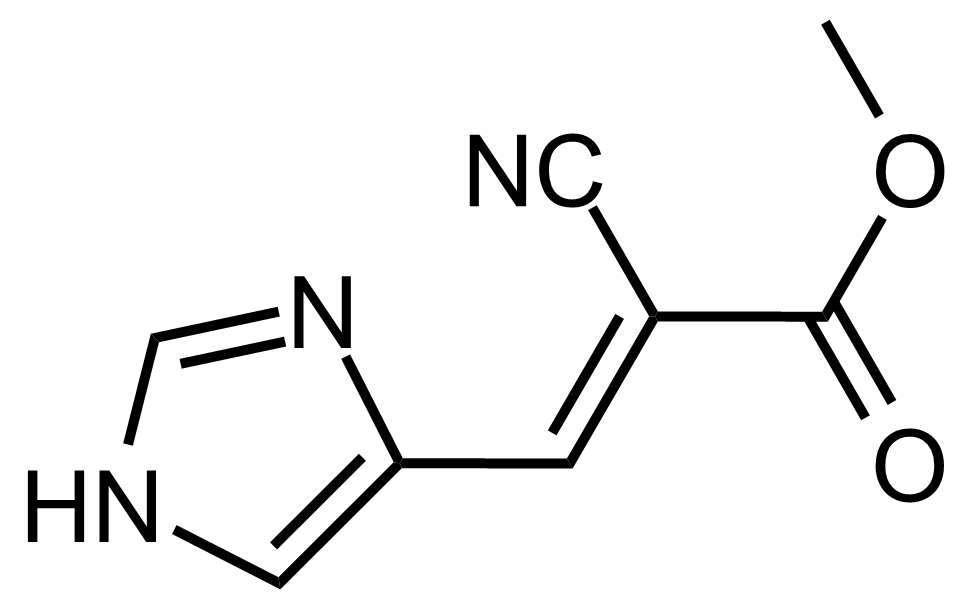 | [] | GEO-03648 |
| (Z)-Methyl 2-cyano-3-(isoquinolin-4-yl)acrylate | 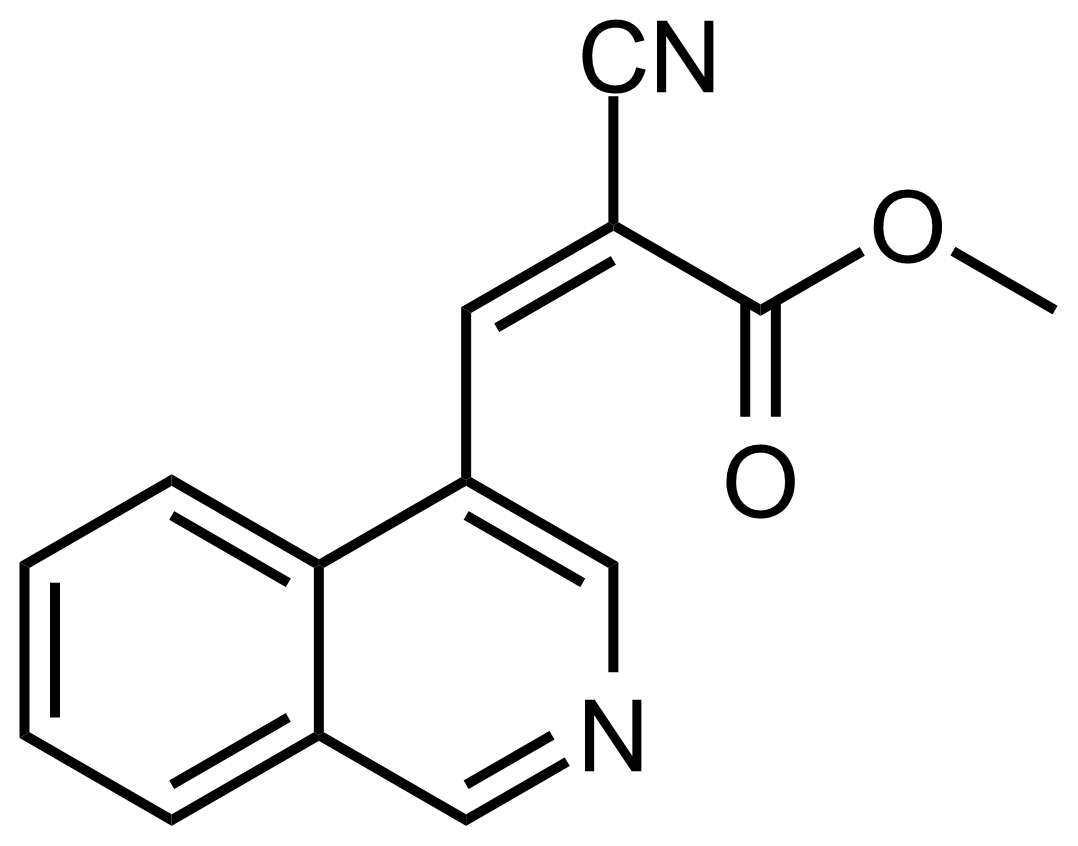 | [] | GEO-03669 |
| (E)-Methyl 2-cyano-3-(2-methoxyphenyl)acrylate | 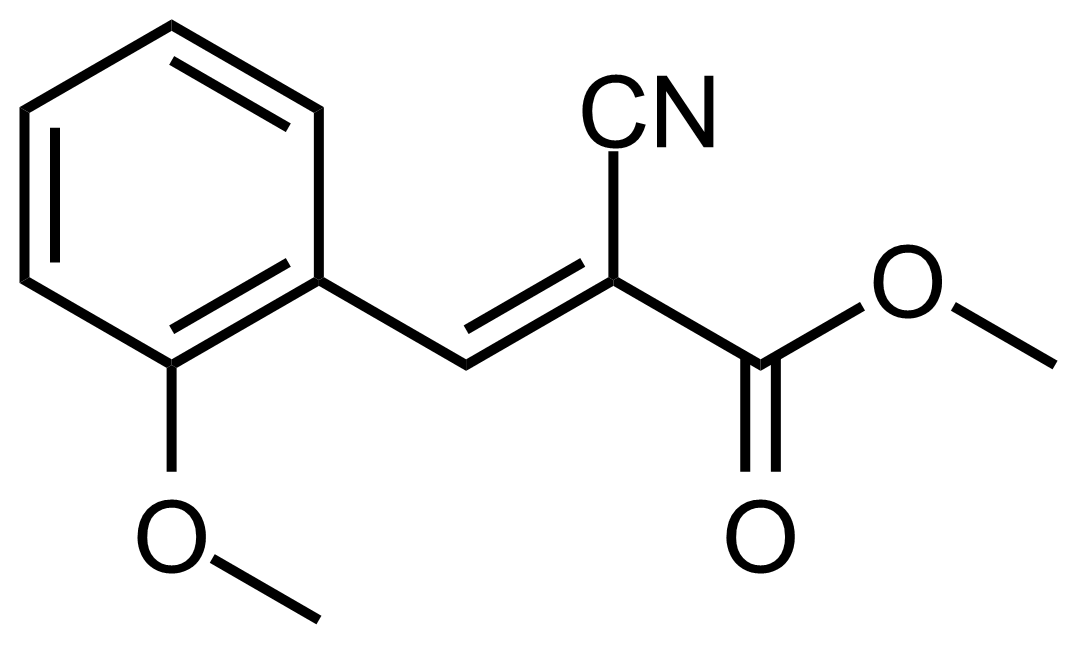 | N/A | GEO-03600 |
| (E)-Methyl 2-cyano-3-(5-(4-methoxyphenyl)thiophen-2-yl)acrylate | 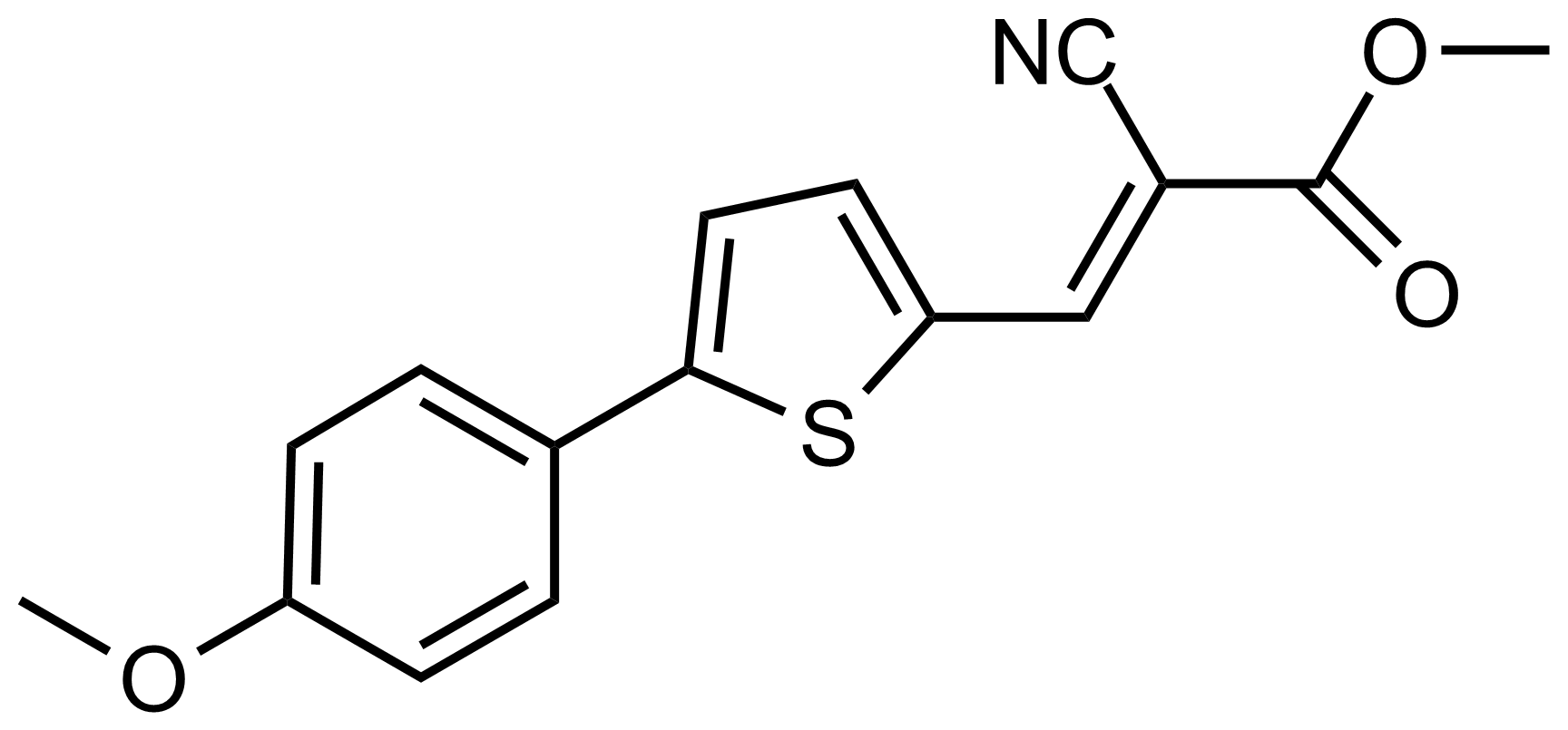 | N/A | GEO-03586 |
| (E)-Methyl 2-cyano-3-(5-methylfuran-2-yl)acrylate | 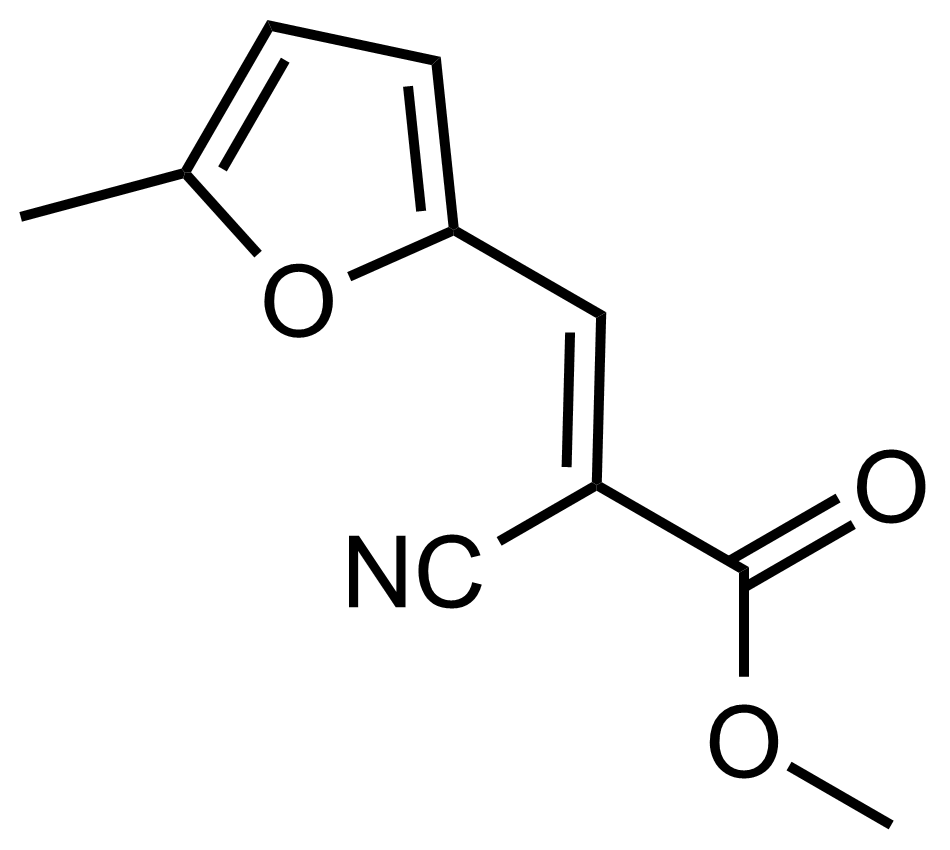 | N/A | GEO-03576 |
| (Z)-Methyl 2-cyano-3-(2-methyl-1H-indol-7-yl)acrylate |  | [] | GEO-03659 |
| (E)-Methyl 2-cyano-3-(6-methylpyridin-3-yl)acrylate | 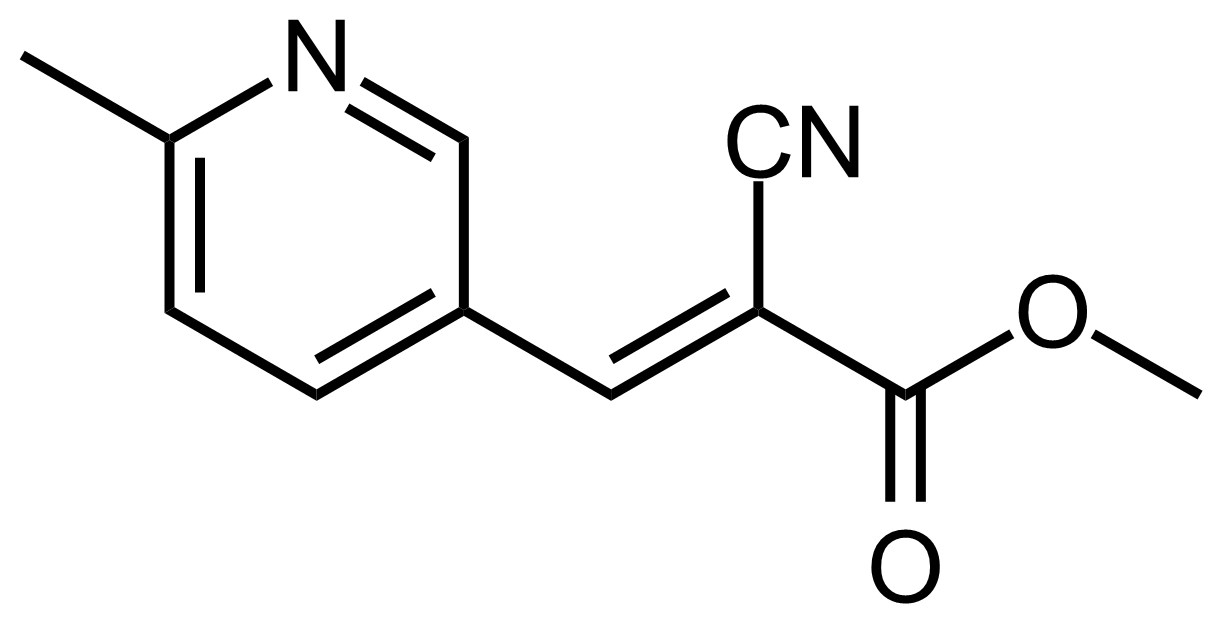 | [] | GEO-03623 |
| (E)-Methyl 2-cyano-3-(2-methylpyridin-3-yl)acrylate | 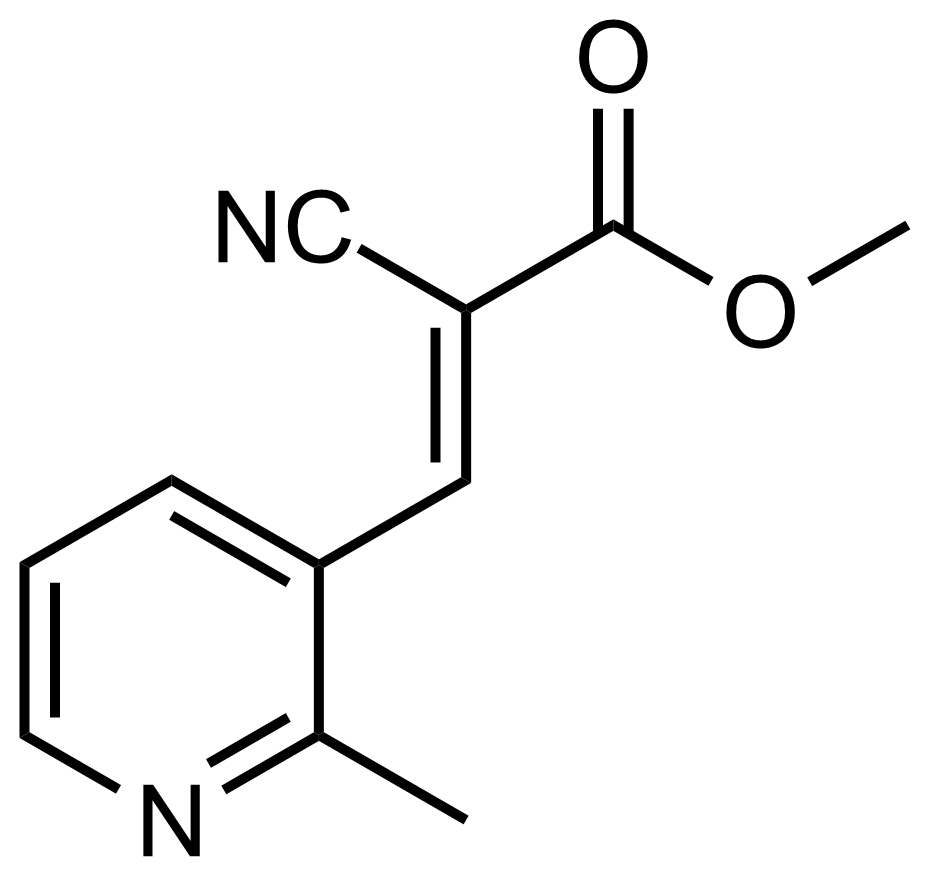 | [] | GEO-03664 |
| (E)-Methyl 2-cyano-3-(3-methylpyridin-2-yl)acrylate | 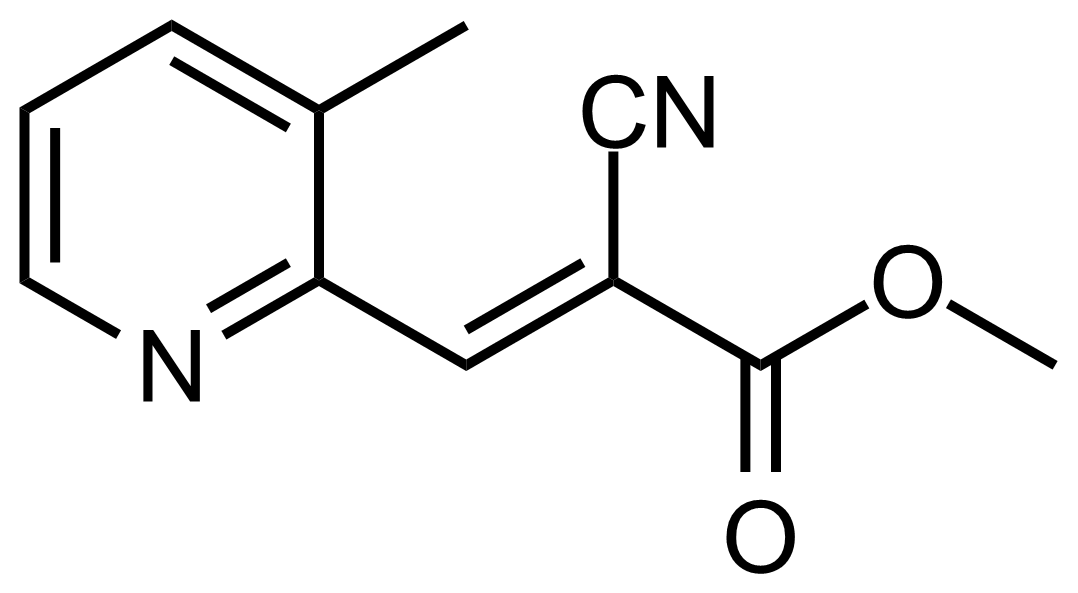 | N/A | GEO-03490 |
| (E)-Methyl 2-cyano-3-(5-methylpyridin-2-yl)acrylate | 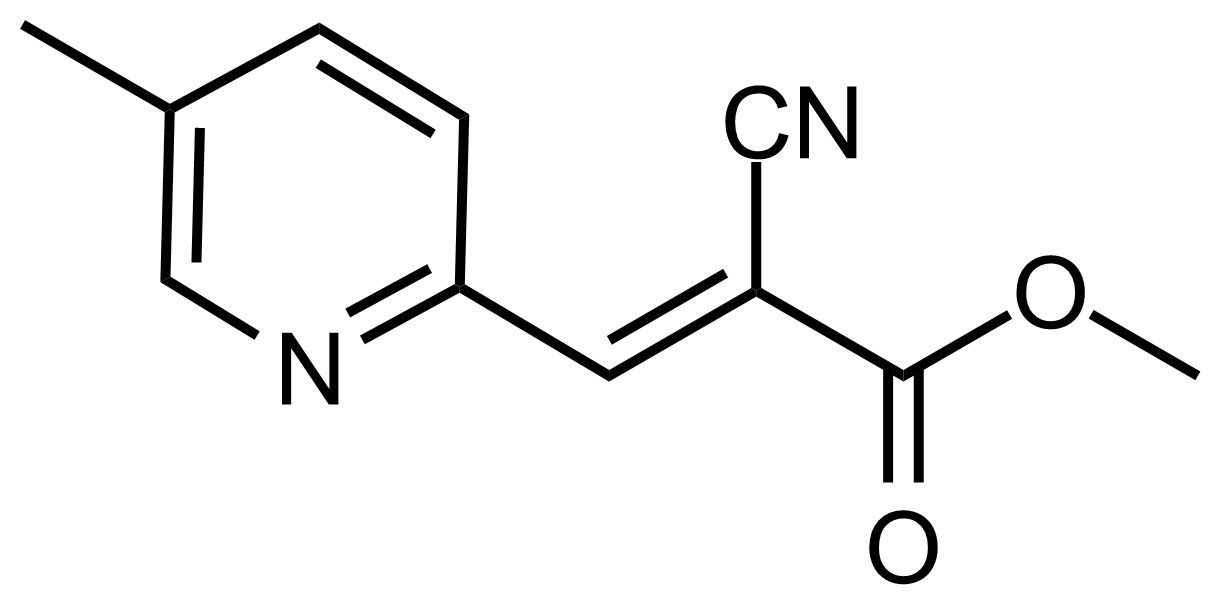 | N/A | GEO-03535 |
| (E)-Methyl 2-cyano-3-(5-((5-methyl-1,3,4-thiadiazol-2-yl)thio)furan-2-yl)acrylate | 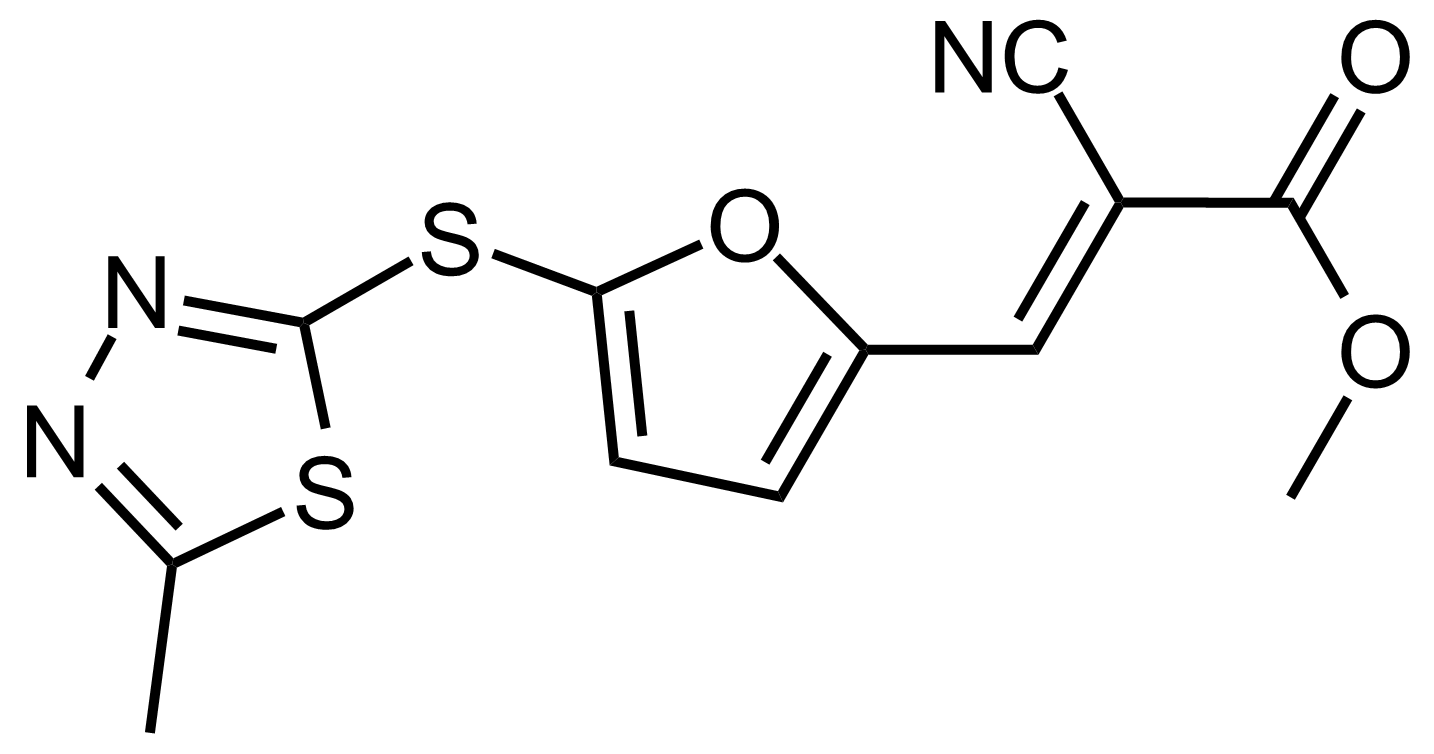 | N/A | GEO-03525 |
| (E)-Methyl 2-cyano-3-(pyridin-2-yl)acrylate | 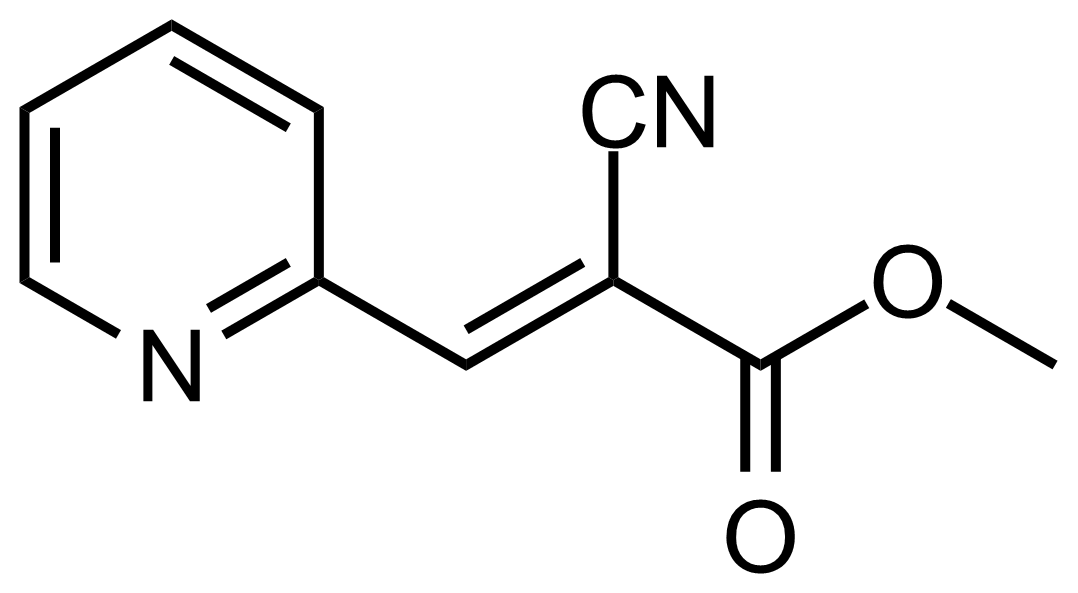 | N/A | GEO-03603 |
| (Z)-Methyl 2-cyano-3-(5-(pyridin-2-ylthio)furan-2-yl)acrylate | 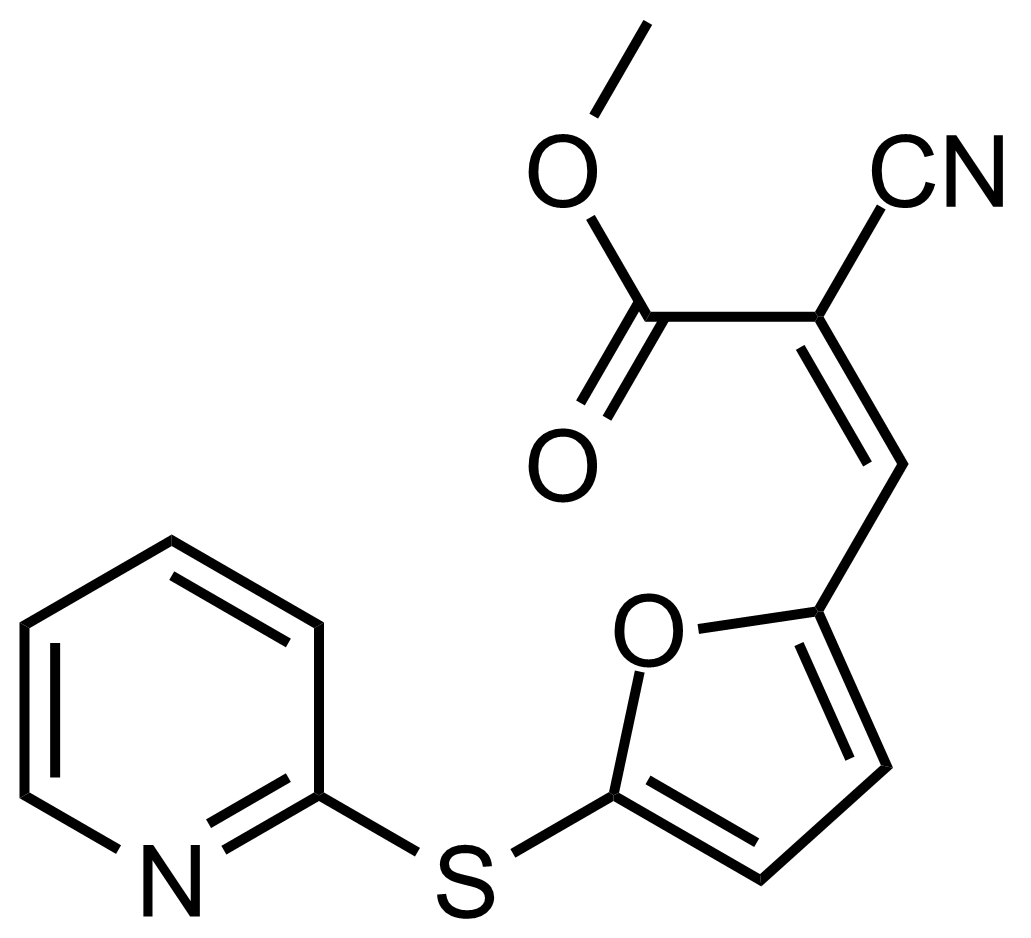 | N/A | GEO-03485 |
| (Z)-Methyl 2-cyano-3-(1H-pyrrolo[2,3-b]pyridin-3-yl)acrylate | 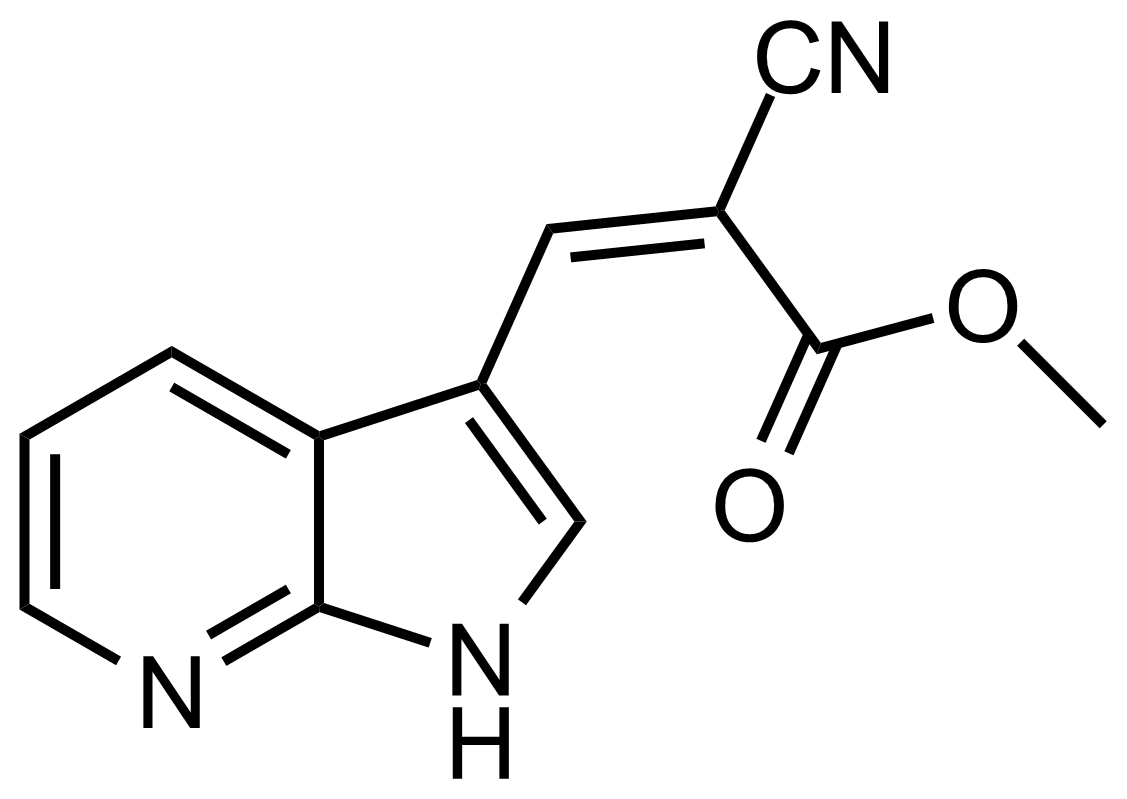 | N/A | GEO-03496 |
| (E)-Methyl 2-cyano-3-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)acrylate | 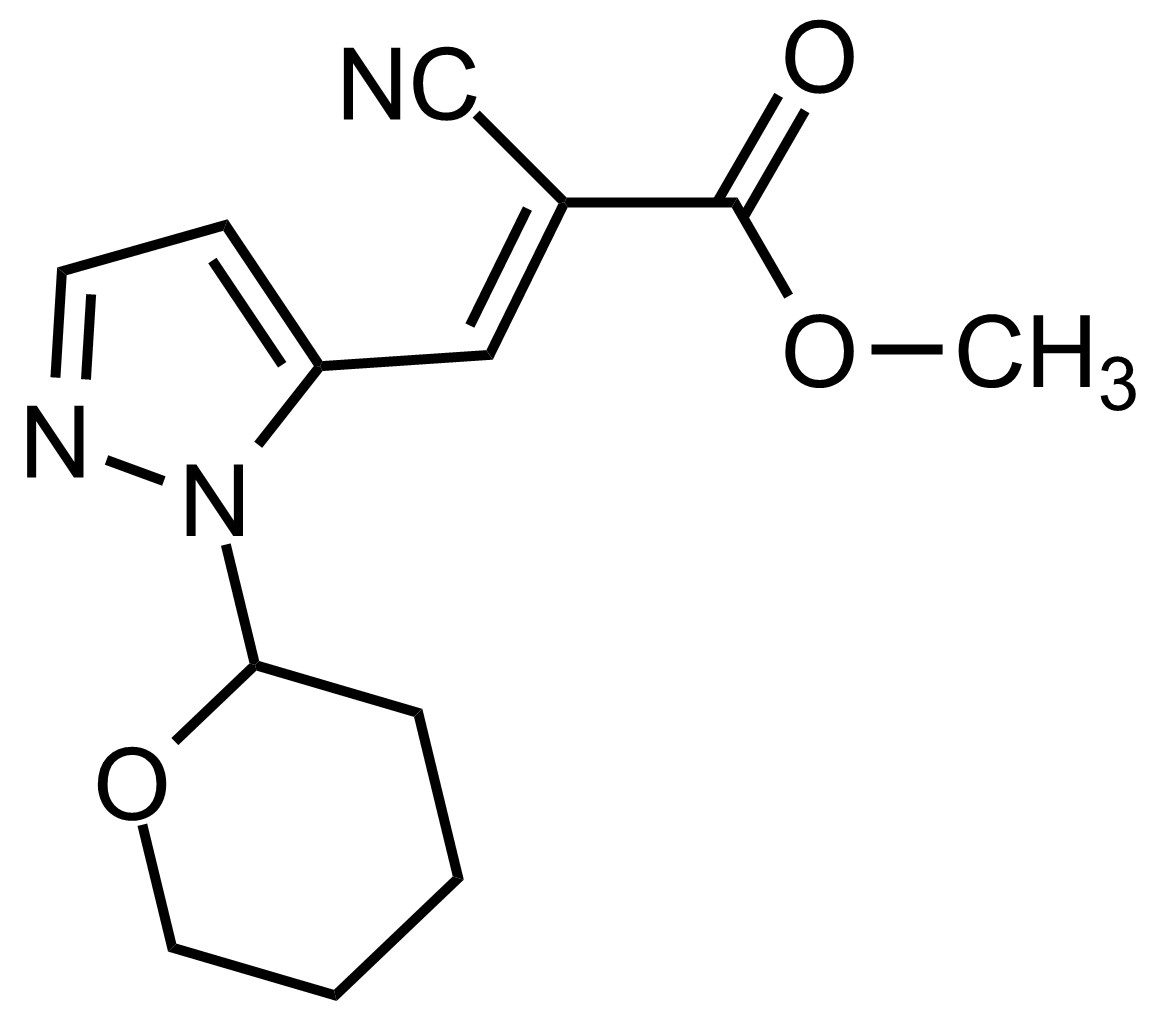 | N/A | GEO-03546 |
| (E)-Methyl 2-cyano-3-(thiophen-3-yl)acrylate | 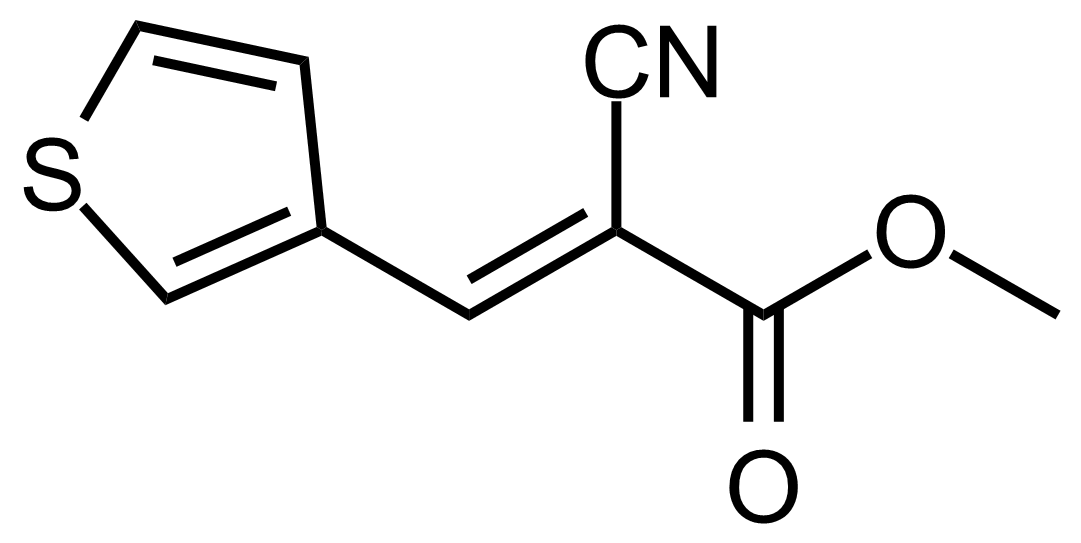 | [] | GEO-03625 |
| Methyl 3-cyclopentenecarboxylate | 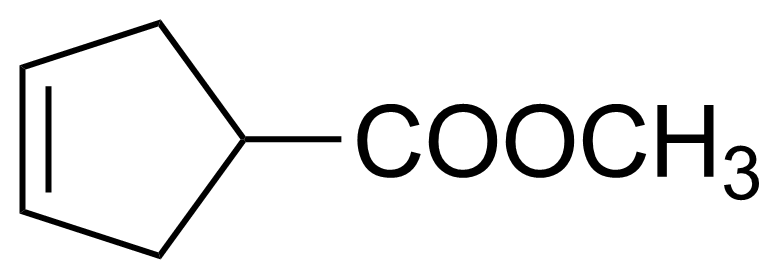 | [58101-60-3] | GEO-02841 |
| Methyl 4-(2,6-difluoro-4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 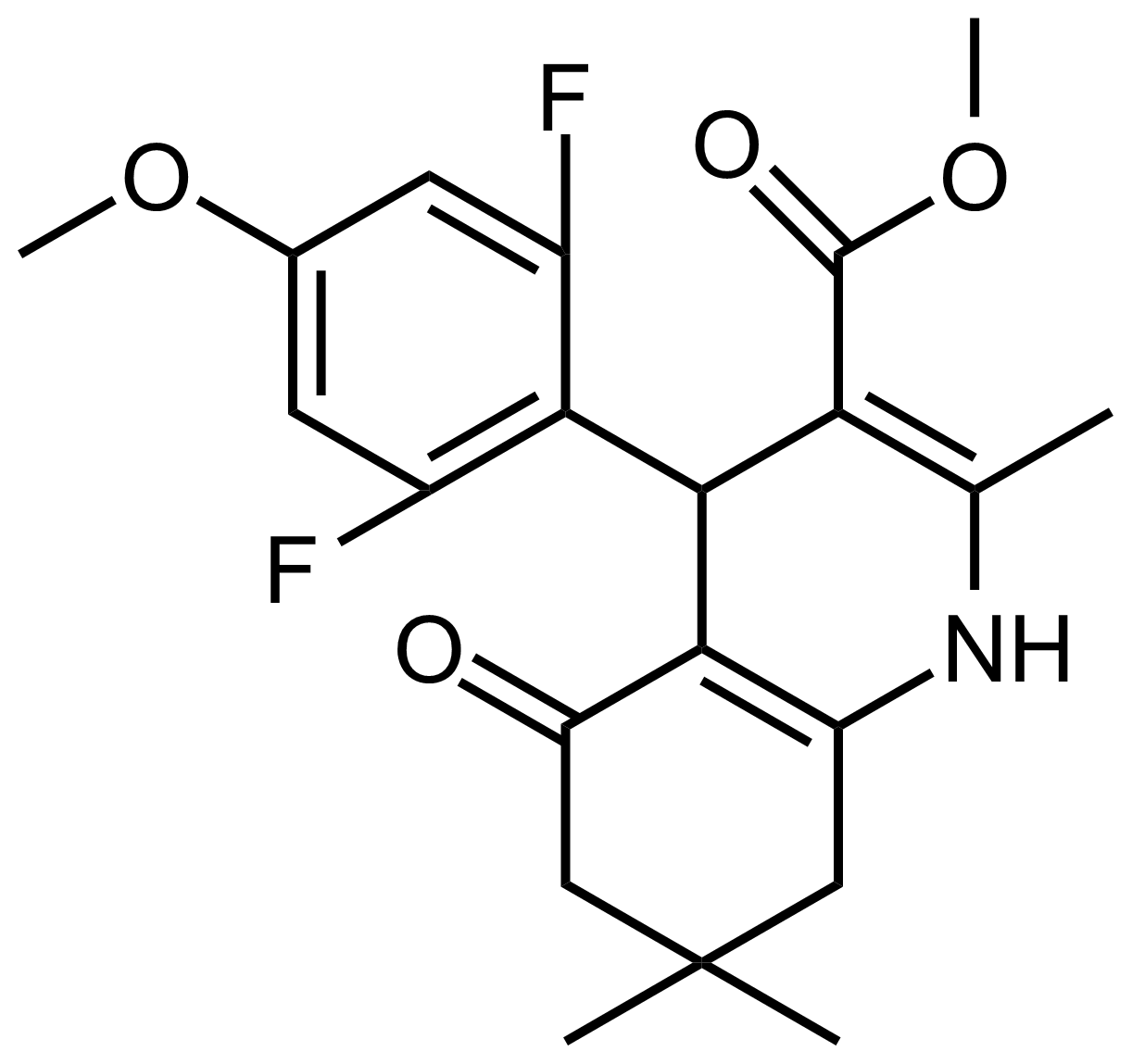 | N/A | GEO-03500 |
| New | Methyl 4,5-dimethoxy-3-hydroxybenzoate | 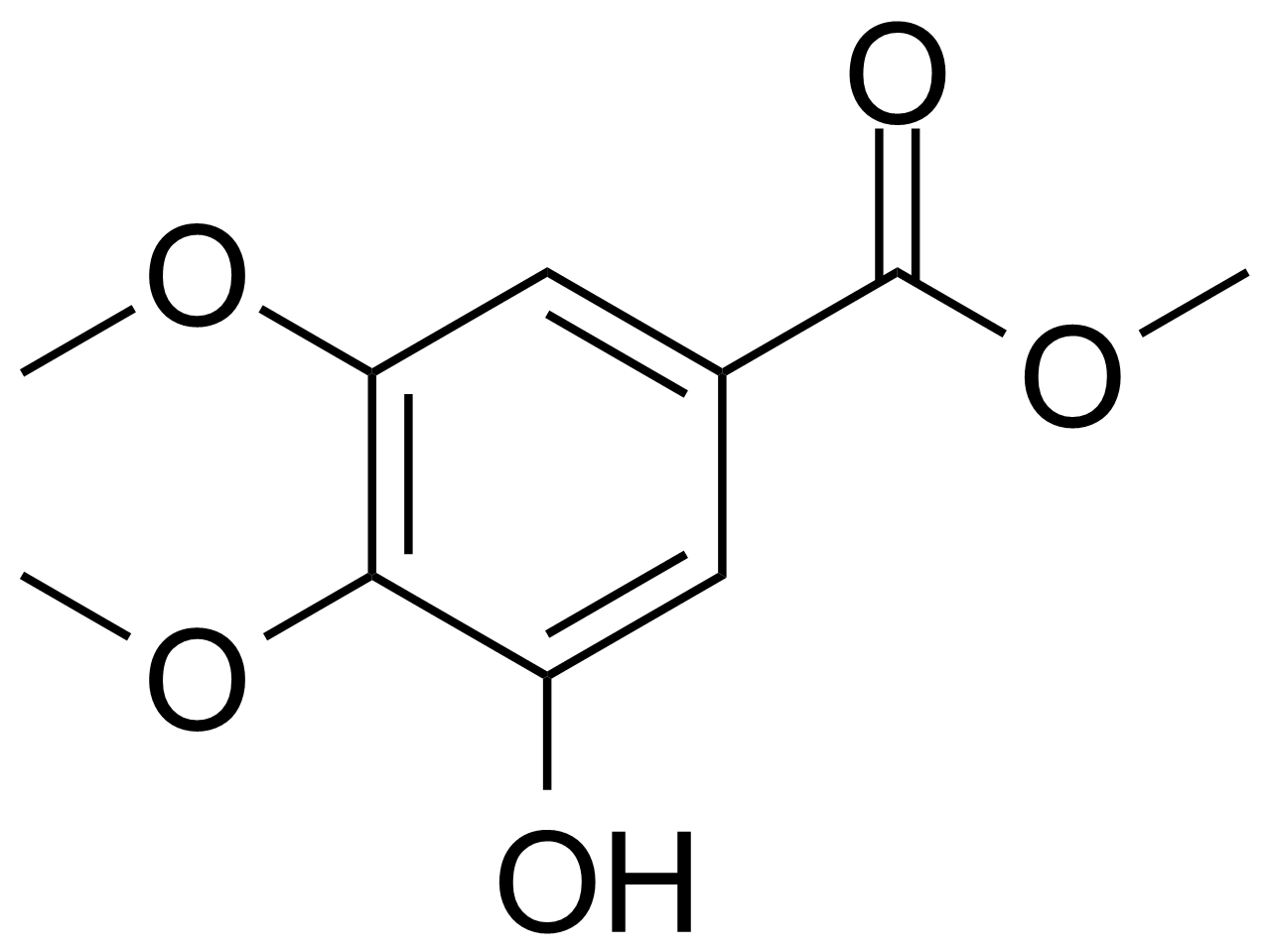 | [83011-43-2] | GEO-01807 |
| Methyl 2,2-dimethyl-1,3-dioxolane-4-carboxylate | 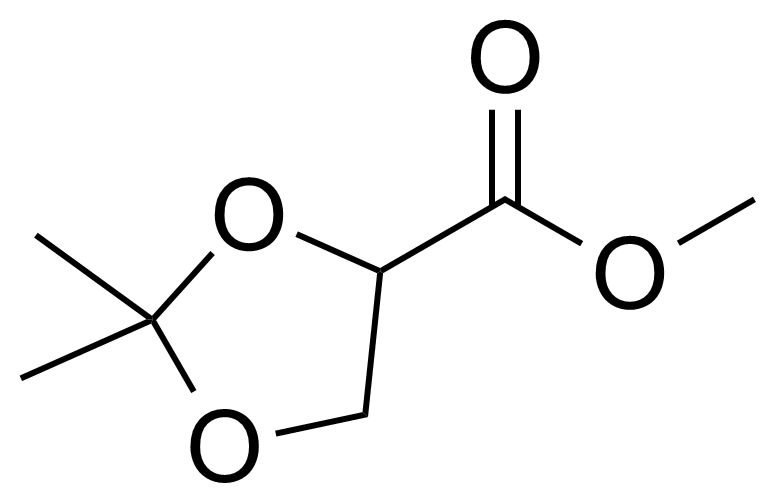 | [108865-84-5] | GEO-03987 |
| a-Methylene-g-butyrolactone | 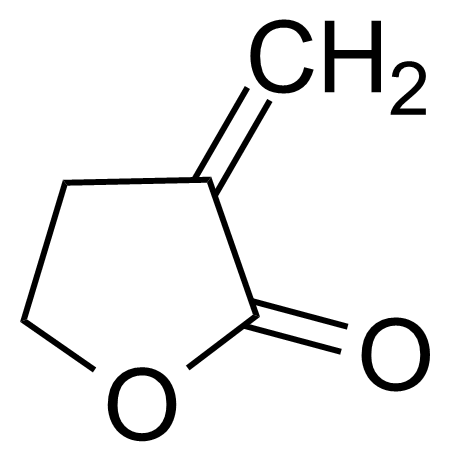 | [547-65-9] | GEO-03235 |
| alpha-Methylene-gamma-valerolactone |  | [62873-16-9] | GEO-04344 |
| Methyl 4-(3-fluorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 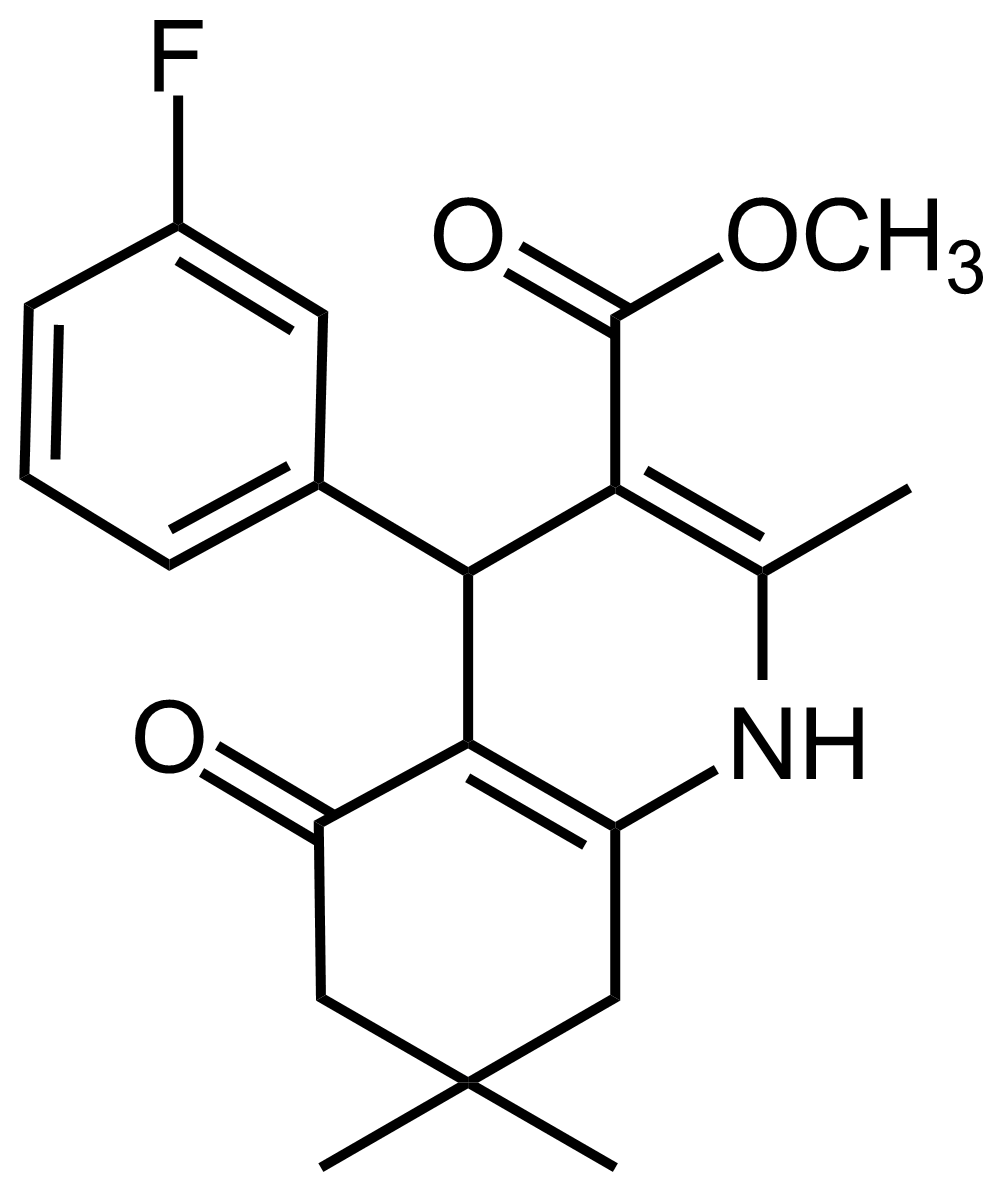 | N/A | GEO-03539 |
| Methyl 4-(4-fluorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 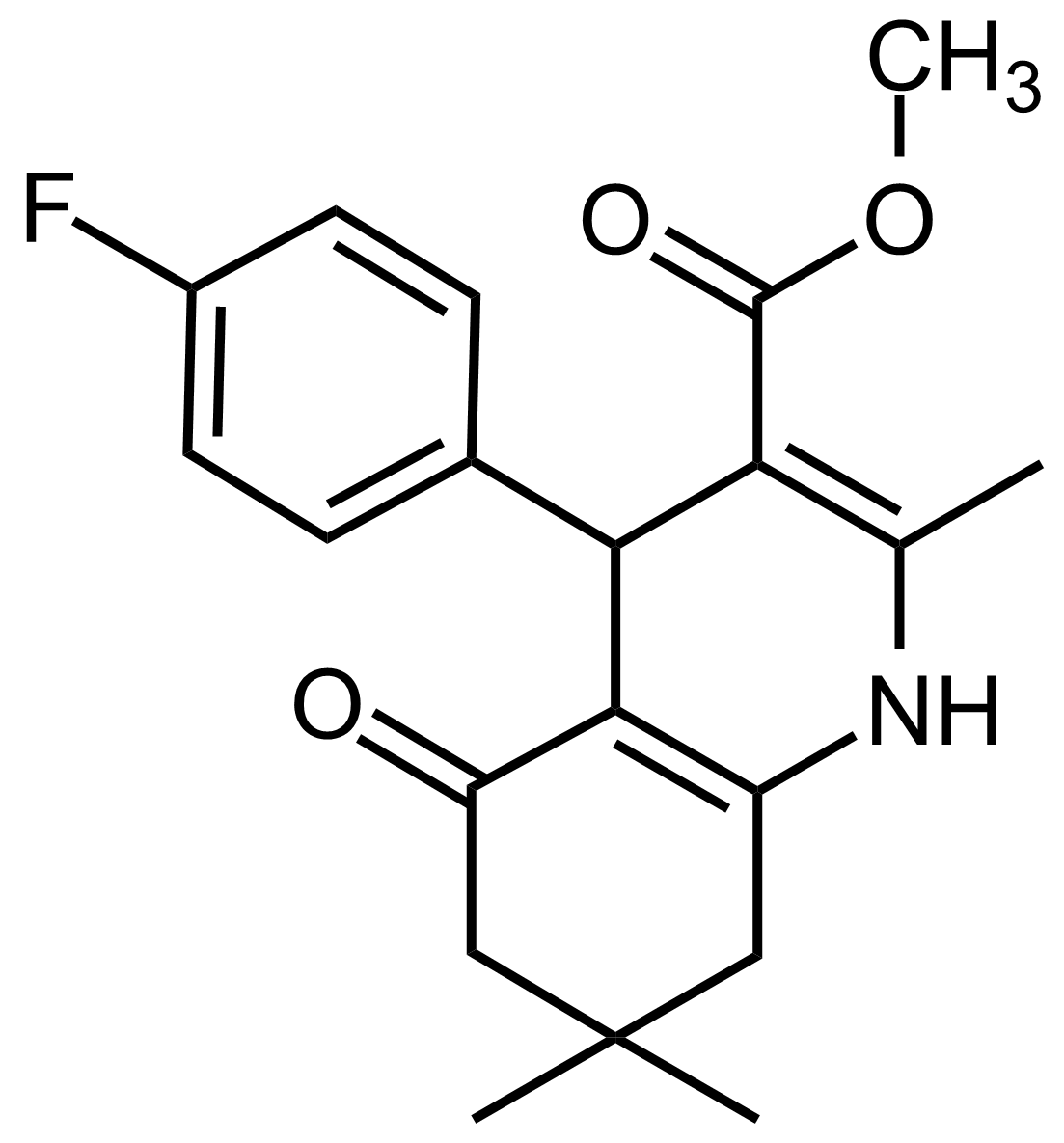 | N/A | GEO-03555 |
| 3-Methyl-2(5H)-furanone | 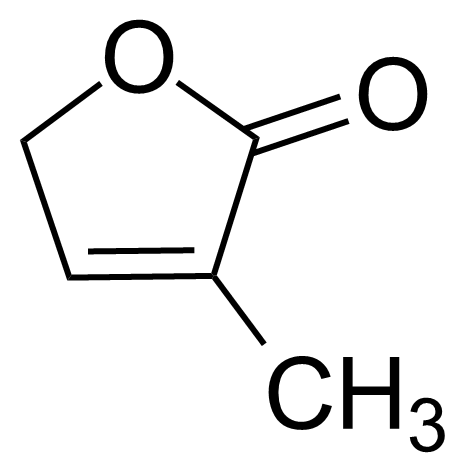 | [22122-36-7] | GEO-01823 |
| Methyl 3-furoate | 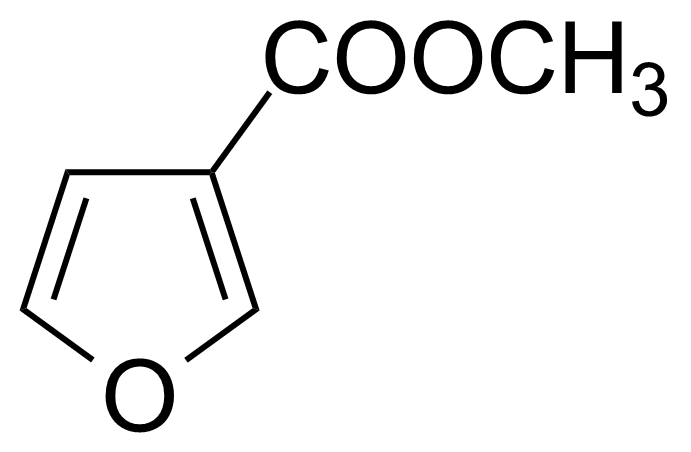 | [13129-23-2] | GEO-03412 |
| Methyl 4-(5-hexylthiophen-2-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 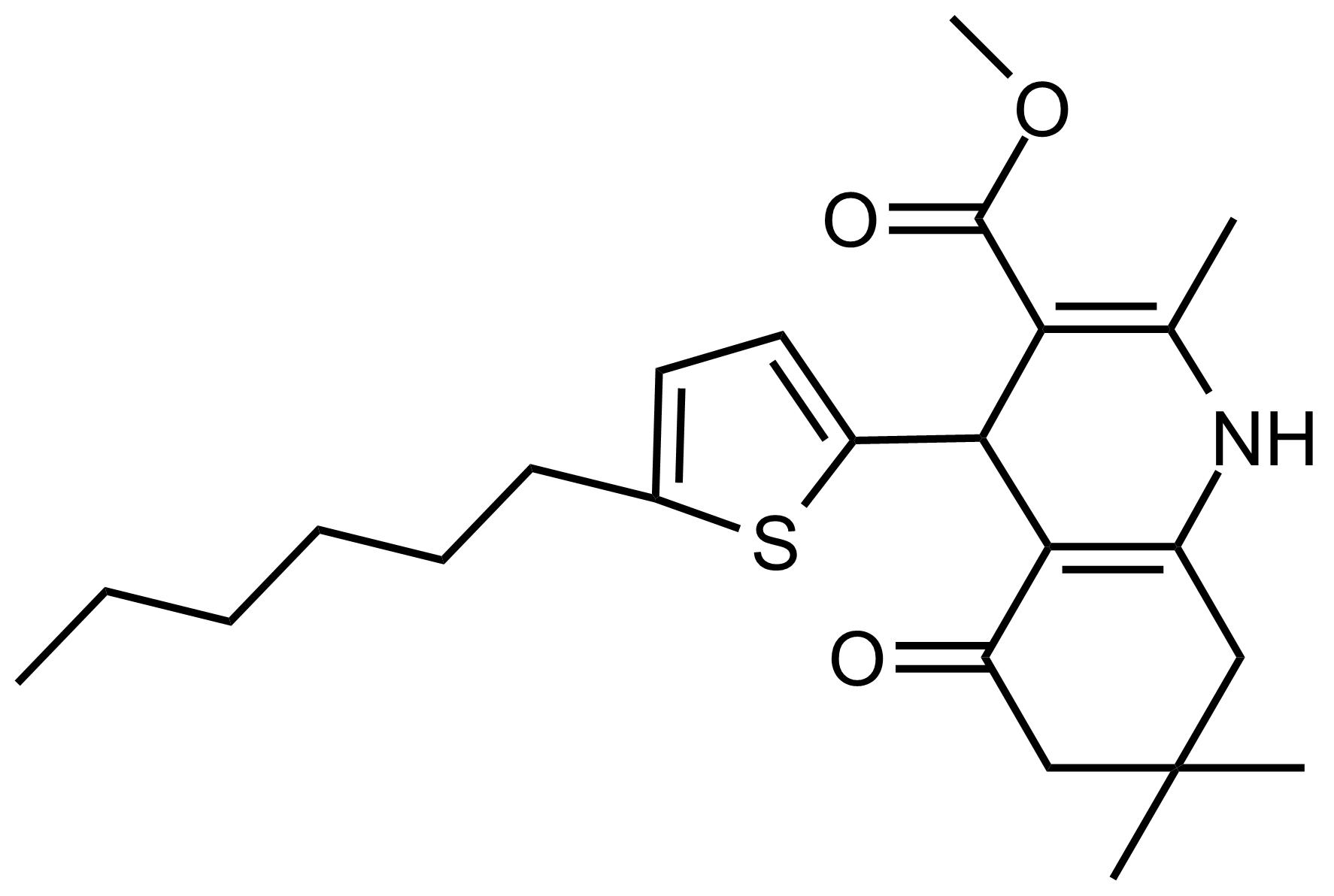 | N/A | GEO-03529 |
| Methyl 3-hydroxyadamantane-1-carboxylate | 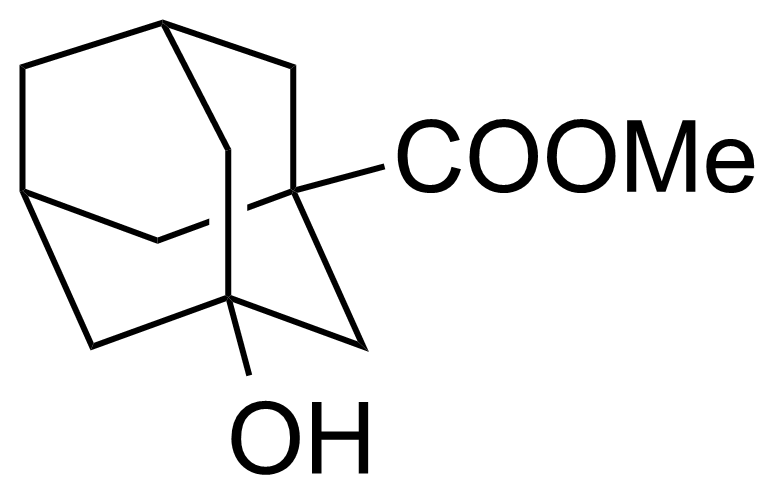 | [68435-07-4] | GEO-04335 |
| (2S,4R)-Methyl 4-hydroxypiperidine-2-carboxylate hydrochloride | 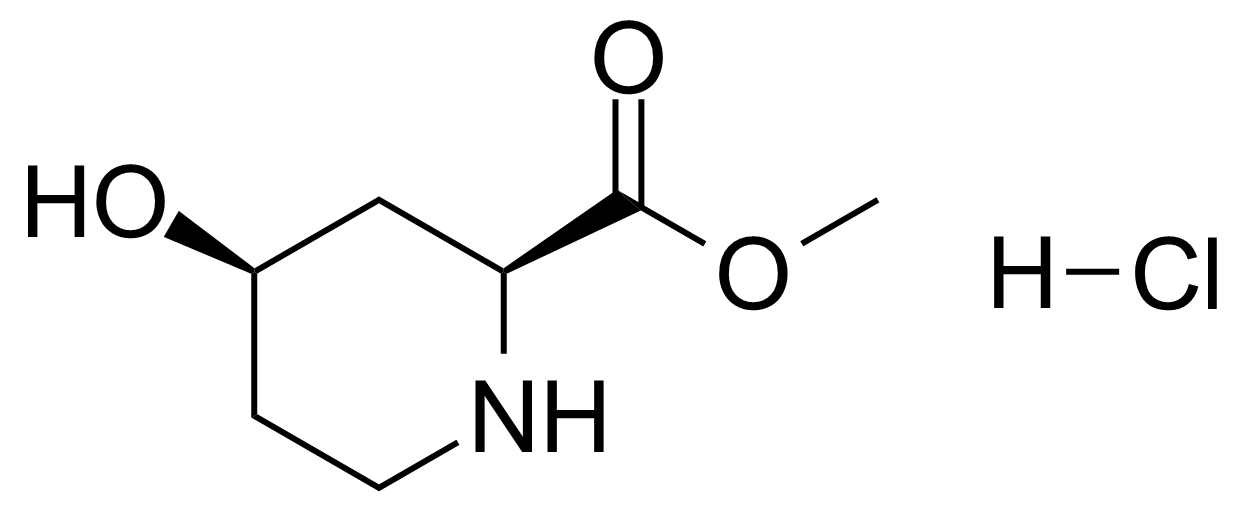 | [175671-43-9] | GEO-03424 |
| Methyl 4-imidazolecarboxylate | 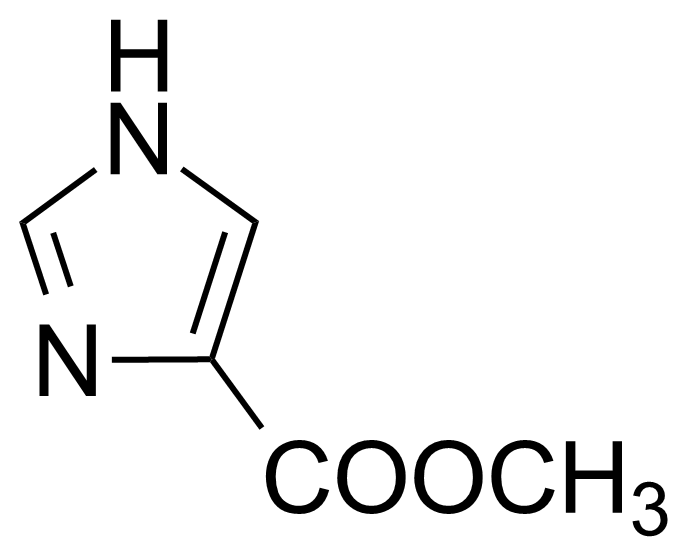 | [17325-26-7] | GEO-01856 |
| Methyl 2-iodobenzoate | 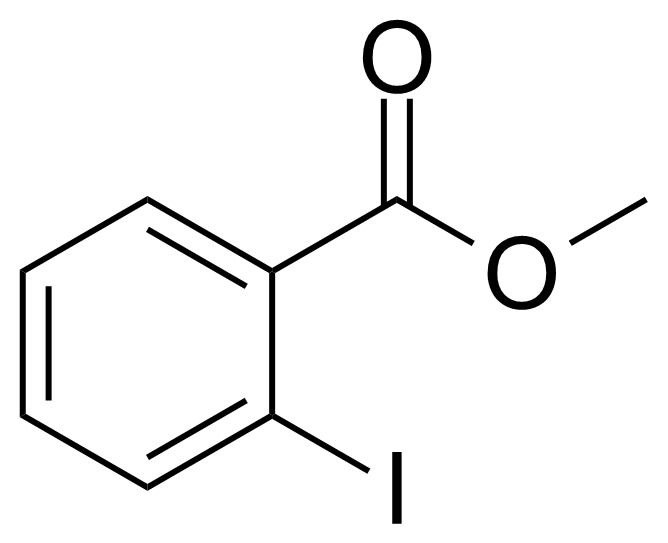 | [610-97-9] | GEO-01859 |
| Methyl 2-isocyanatobenzoate | 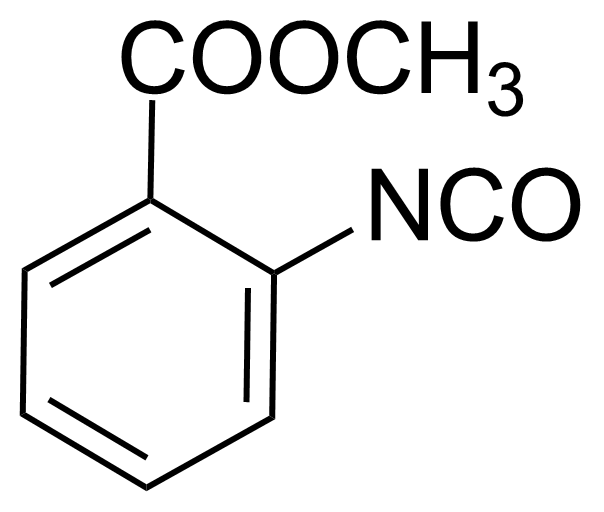 | [1793-07-3] | GEO-01860 |
| Methyl 2-isocyanoacetate | 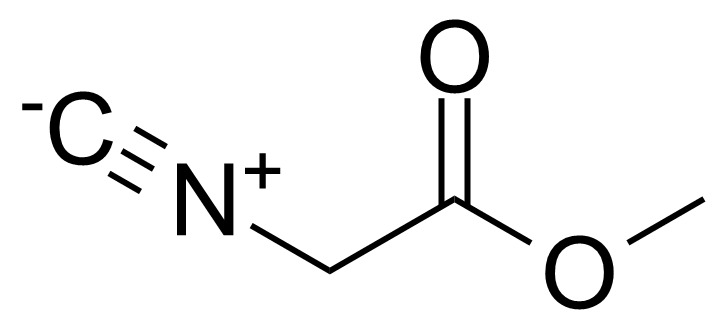 | [39687-95-1] | GEO-03019 |
| Methyl isoxazole-5-carboxylate | 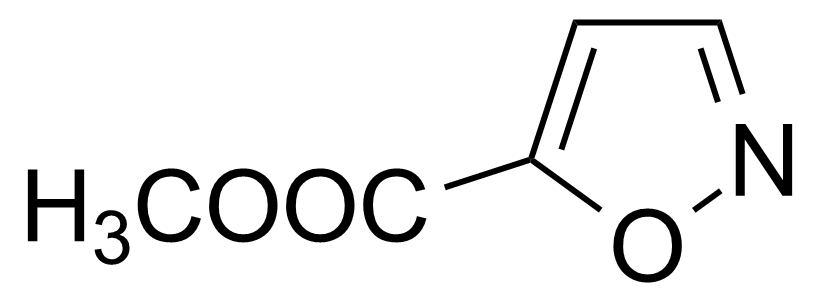 | [15055-81-9] | GEO-02776 |
| Methyl 4-[(4-methoxycarbonylphenyl)carbamoylamino]benzoate | ![Structure of Methyl 4-[(4-methoxycarbonylphenyl)carbamoylamino]benzoate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04233_Methyl_4-4-methoxycarbonylphenylcarbamoylaminobenzoate.png) | [56050-99-8] | GEO-04233 |
| Methyl 3-(4-methoxyphenyl)propionate | 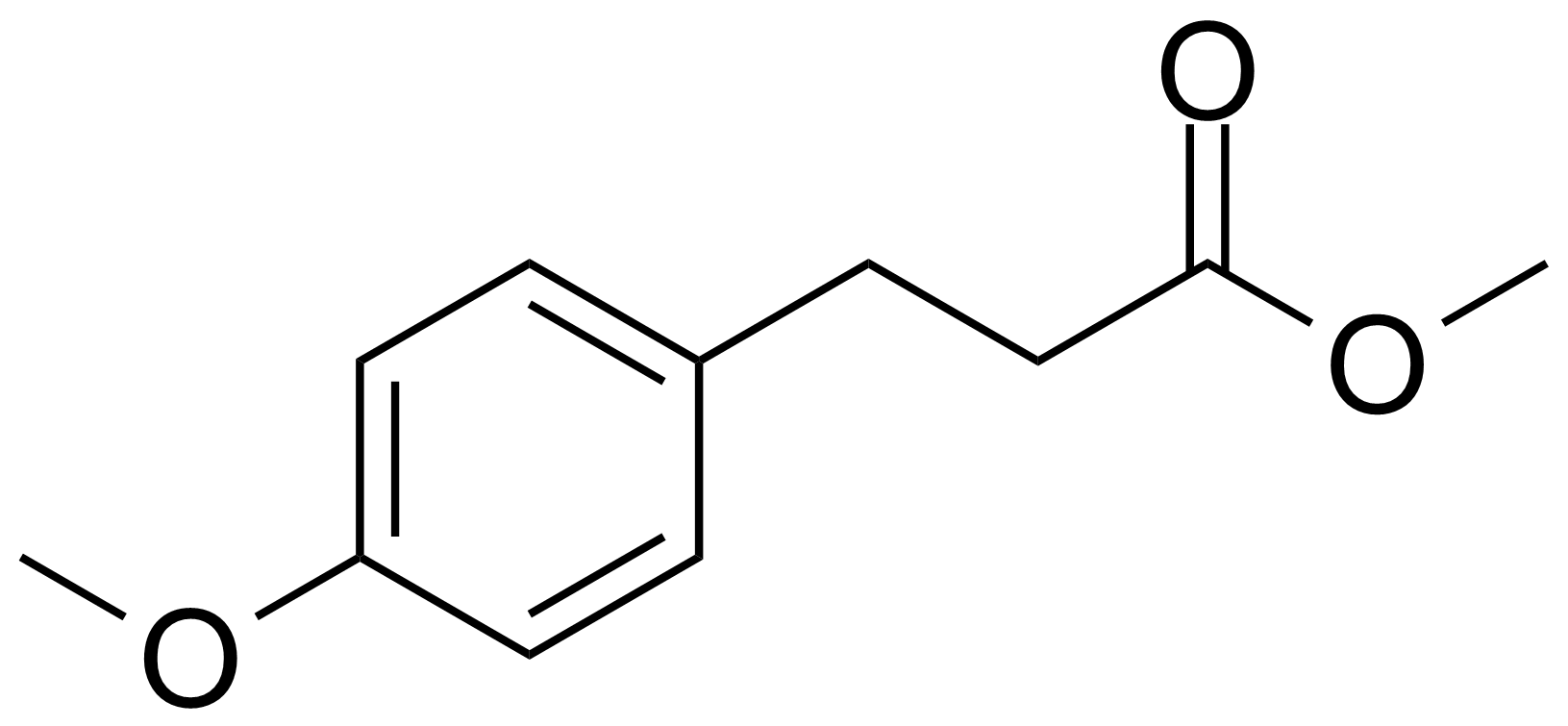 | [15823-04-8] | GEO-01869 |
| Methyl 6-methylbenzo[d]thiazole-2-carboxylate | ![Structure of Methyl 6-methylbenzo[d]thiazole-2-carboxylate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04028_Methyl_6-methylbenzodthiazole-2-carboxylate.png) | [1236115-18-6] | GEO-04028 |
| Methyl 5-methylbenzo[d]thiazole-2-carboxylate | ![Structure of Methyl 5-methylbenzo[d]thiazole-2-carboxylate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04027_Methyl_5-methylbenzodthiazole-2-carboxylate.png) | [1323408-10-1] | GEO-04027 |
| Methyl 2-methyl-1,3-benzothiazole-5-carboxylate | 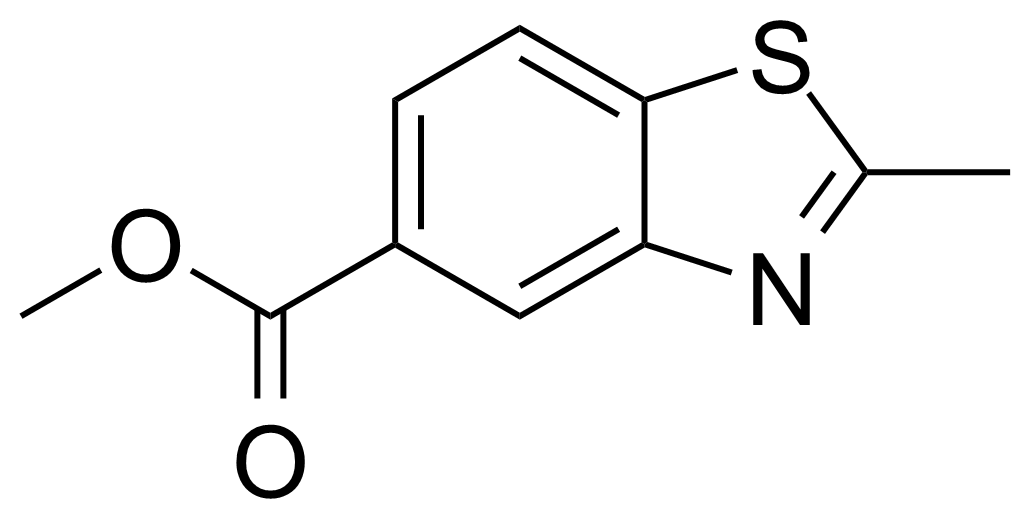 | [32770-98-2] | GEO-01871 |
| Methyl 3-methyl-2-furoate | 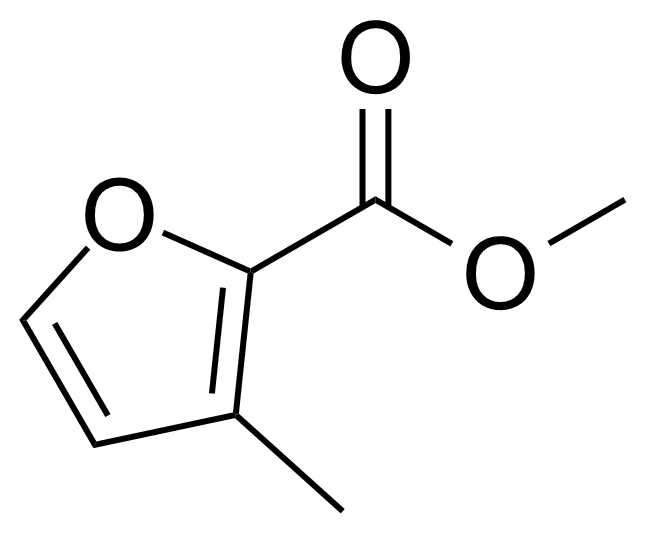 | [6141-57-7] | GEO-01874 |
| Methyl 5-methyl-2-furoate |  | [2527-96-0] | GEO-01875 |
| Methyl 5-nitro-2-furoate | 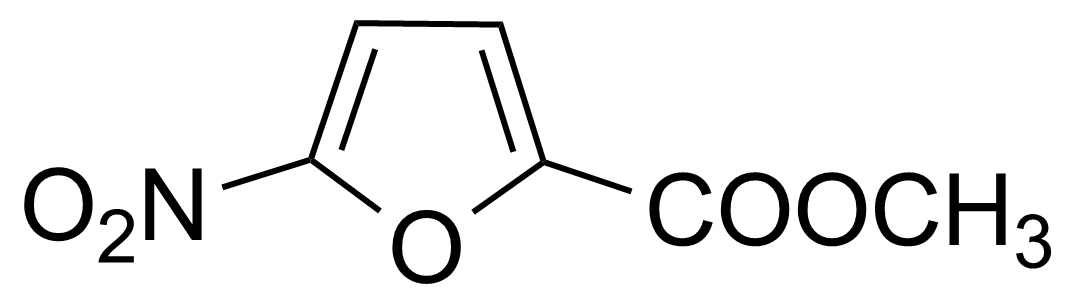 | [1874-23-3] | GEO-01894 |
| Methyl 2′-(5-nitrothiophen-2-yl)-1H,3’H-[2,5′-bibenzo[d]imidazole]-6-carboxylate | ![Structure of Methyl 2'-(5-nitrothiophen-2-yl)-1H,3'H-[2,5'-bibenzo[d]imidazole]-6-carboxylate](https://georganics.sk/wp-content/uploads/2021/06/GEO-01900_Methyl_2-5-nitrothiophen-2-yl-1H3H-25-bibenzodimidazole-6-carboxylate.png) | [N/A] | GEO-01900 |
| Methyl 4-oxo-1-adamantanecarboxylate | 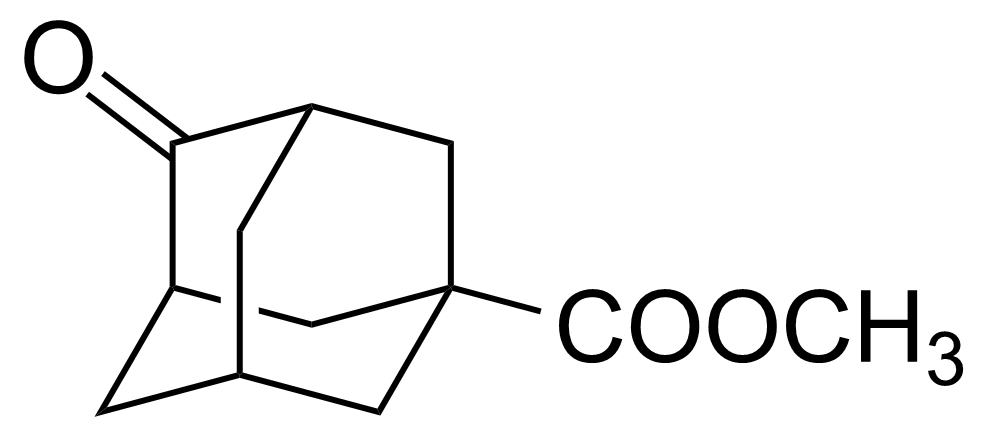 | [56674-88-5] | GEO-03028 |
| Methyl 1H-pyrazole-3-carboxylate | 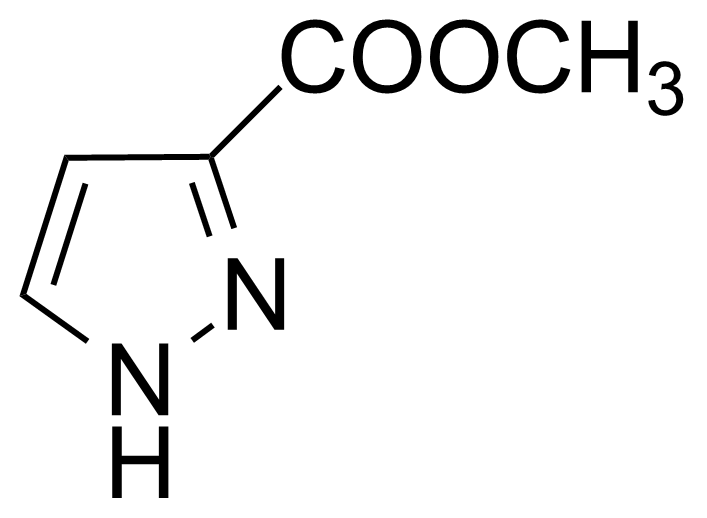 | [15366-34-4] | GEO-01919 |
| Methyl 2-(4-(1H-pyrrol-1-yl)phenoxy)acetate | 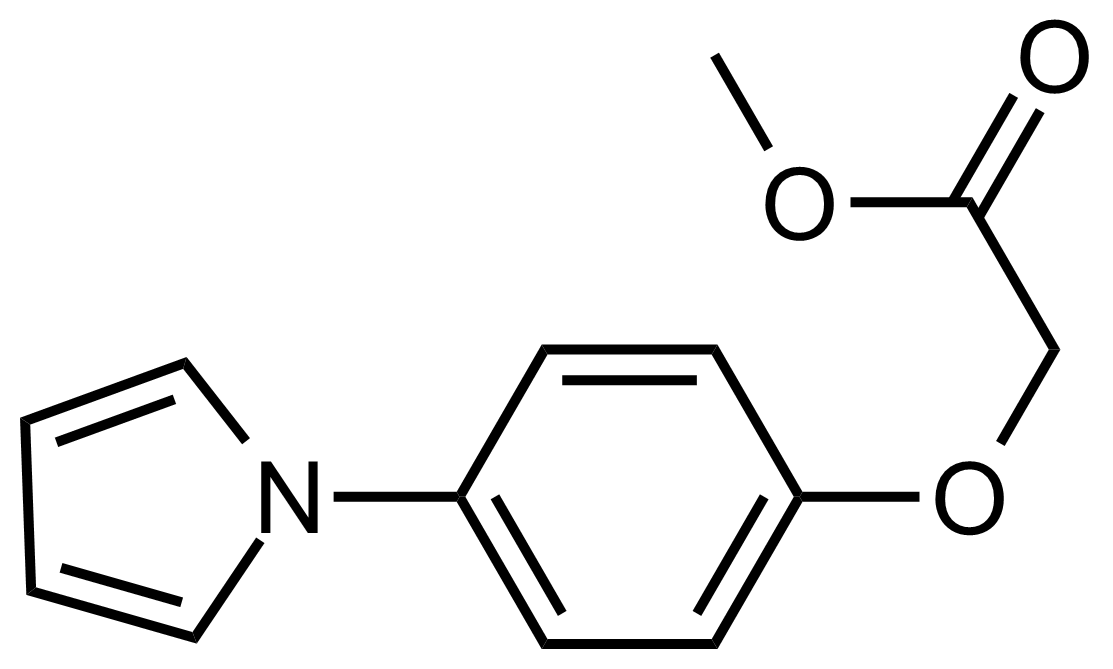 | N/A | GEO-03487 |
| Methyl 2-(thiophene-2-carboxamido)acetate | 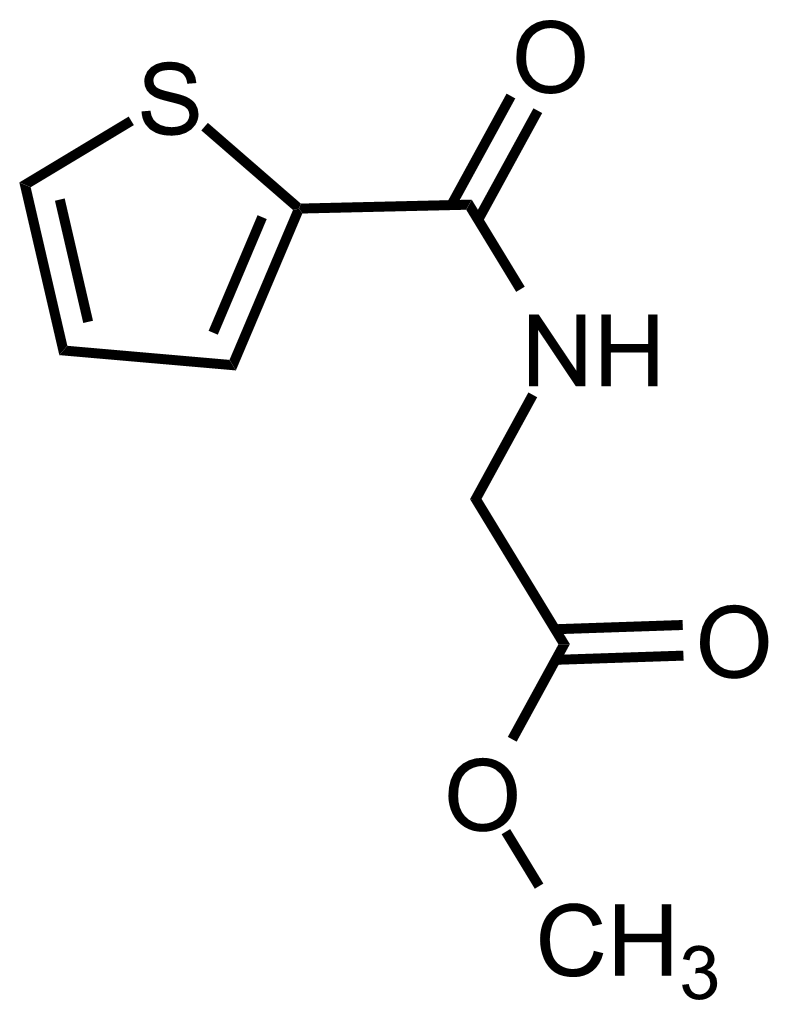 | N/A | GEO-03556 |
| Methyl 3-thiophenecarboxylate | 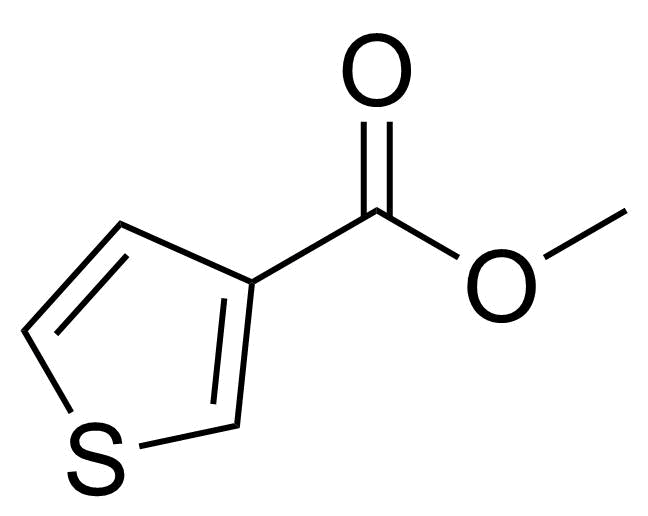 | [22913-26-4] | GEO-04563 |
| Methyl 2,7,7-trimethyl-4-(3-methylpyridin-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 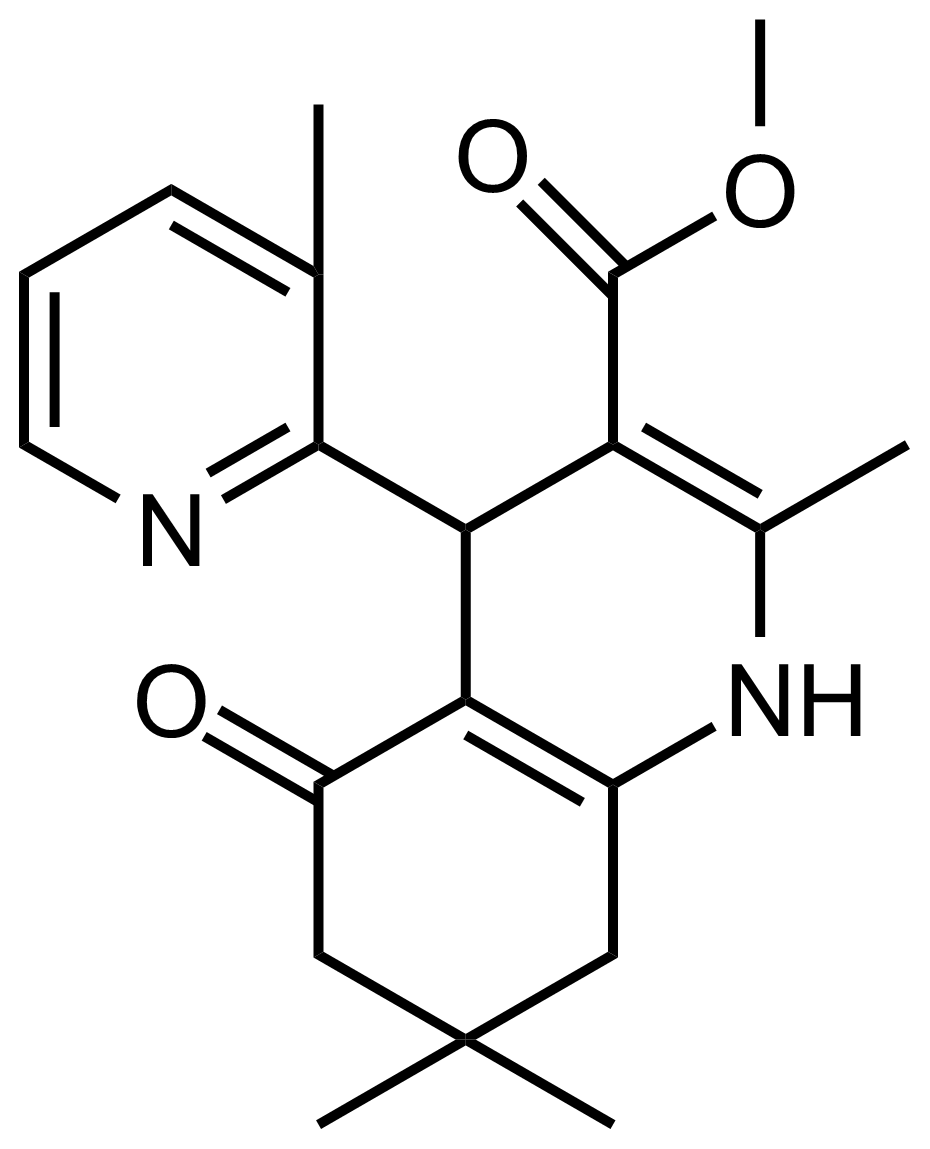 | N/A | GEO-03497 |
| Monobenzylmalonate | 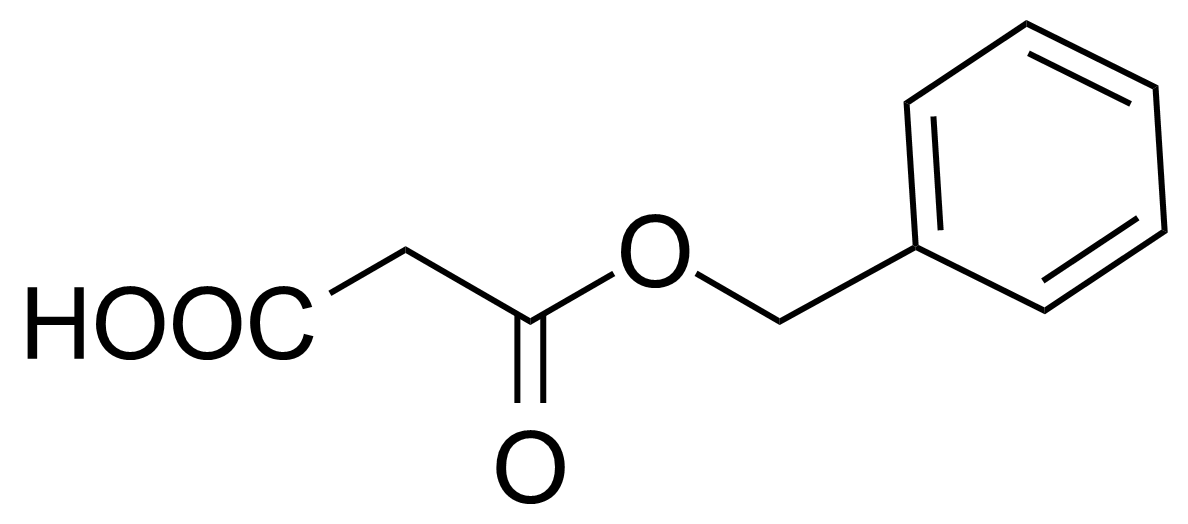 | [40204-26-0] | GEO-01982 |
| New | 4-Nitrophenyl trans-ferulate | 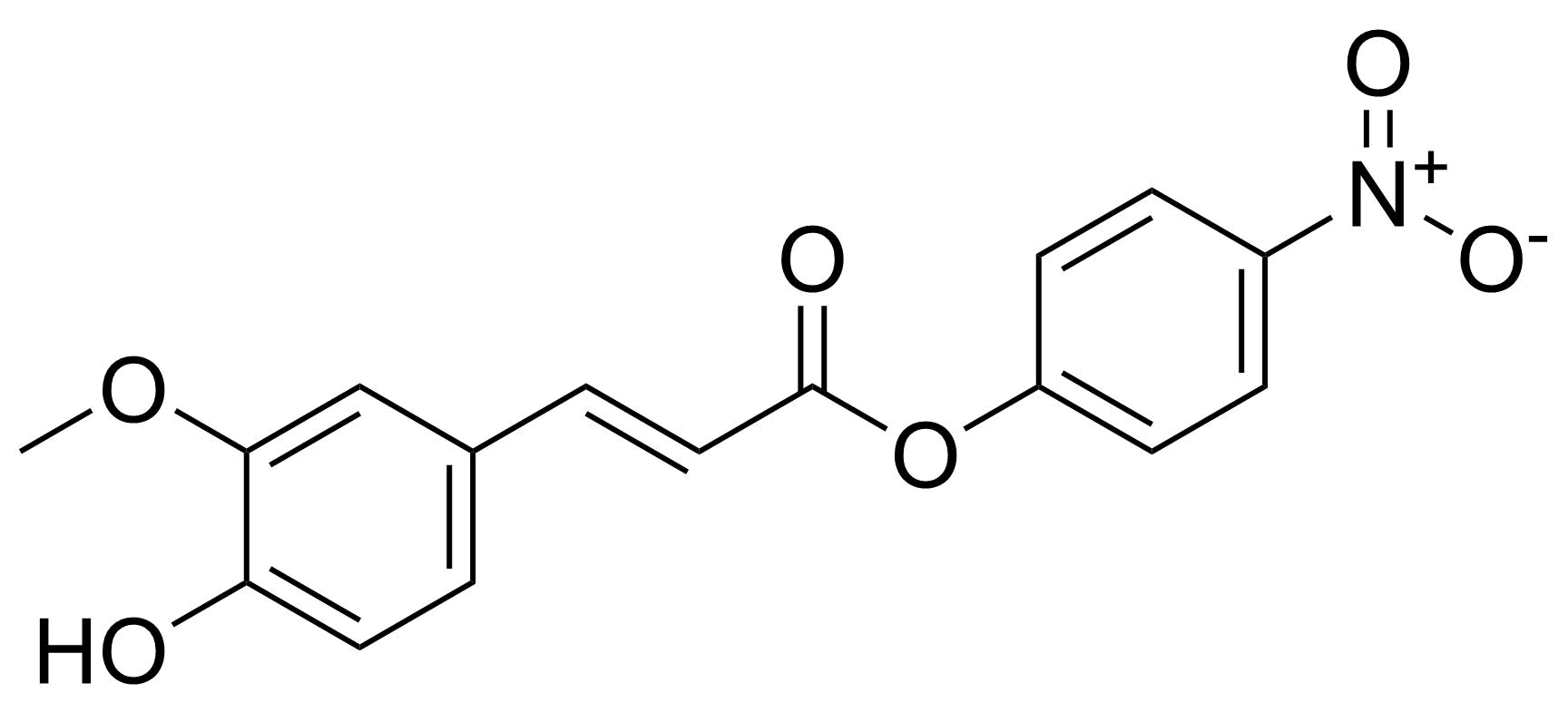 | [398128-60-4] | GEO-04698 |
| New | p-Nitrophenyl 5-o-trans-Feruloyl-alpha-L-arabinofuranoside | 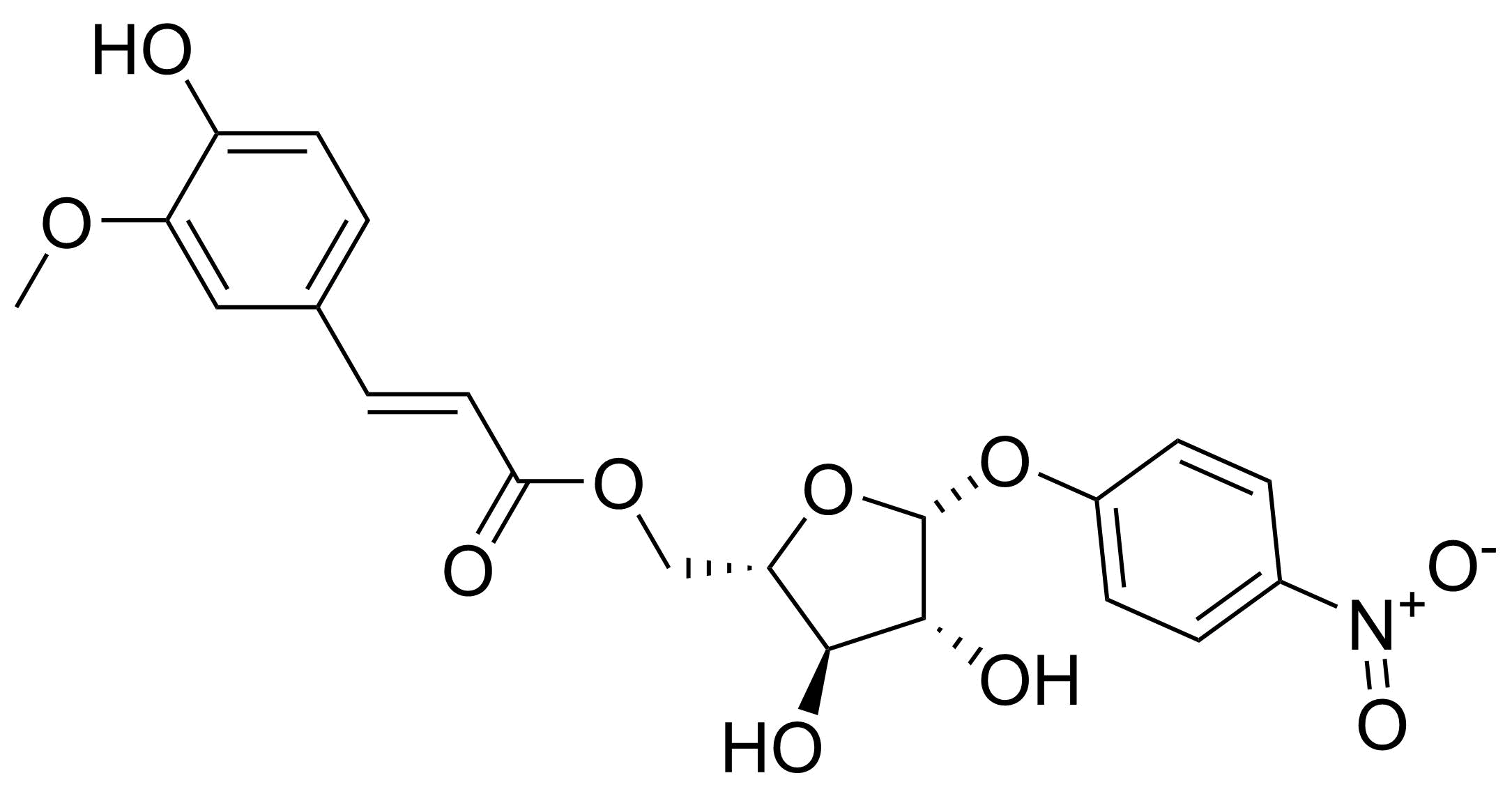 | [508220-79-9] | GEO-04699 |
| 2-Phenyl-1,3-thiazole-4,5-dicarboxylic acid diethylester | 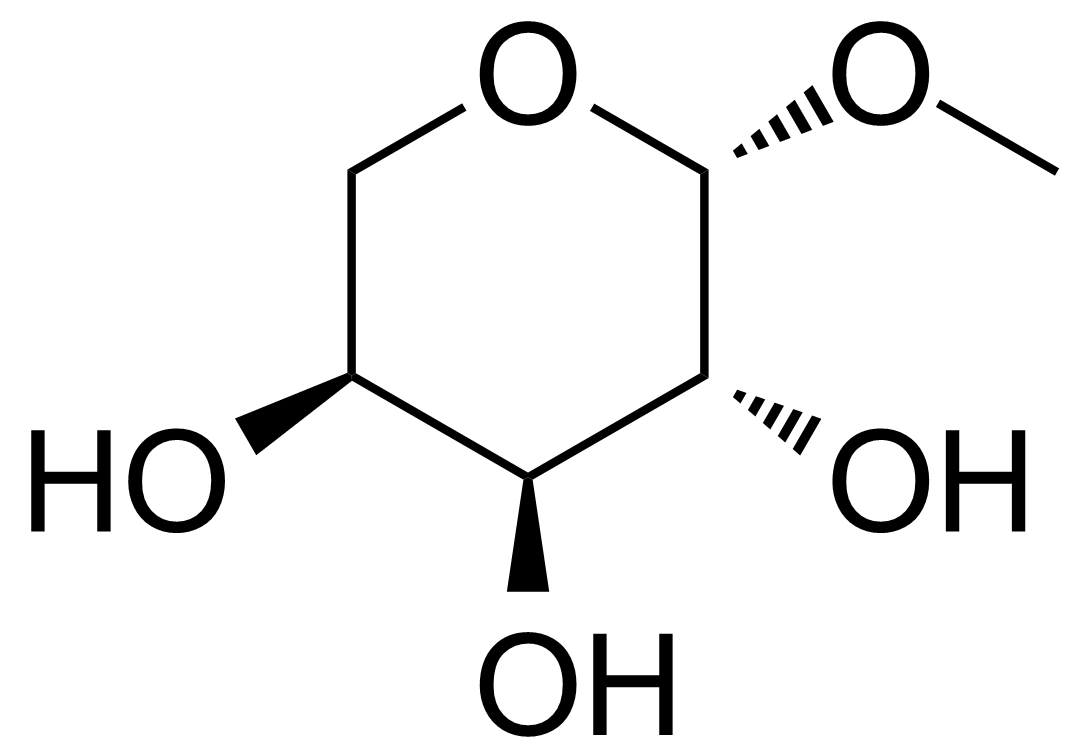 | [54986-96-8] | GEO-02139 |
| 2H-Pyran-2-one | 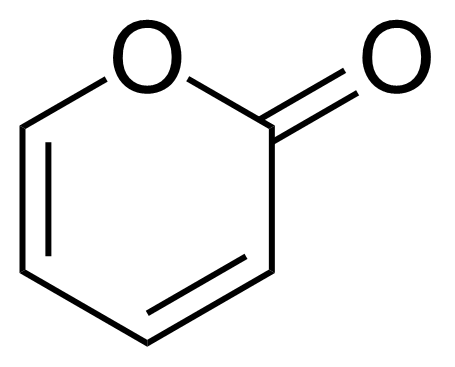 | [504-31-4] | GEO-04164 |
| a-D-Ribofuranose 1,3,5-tribenzoate | 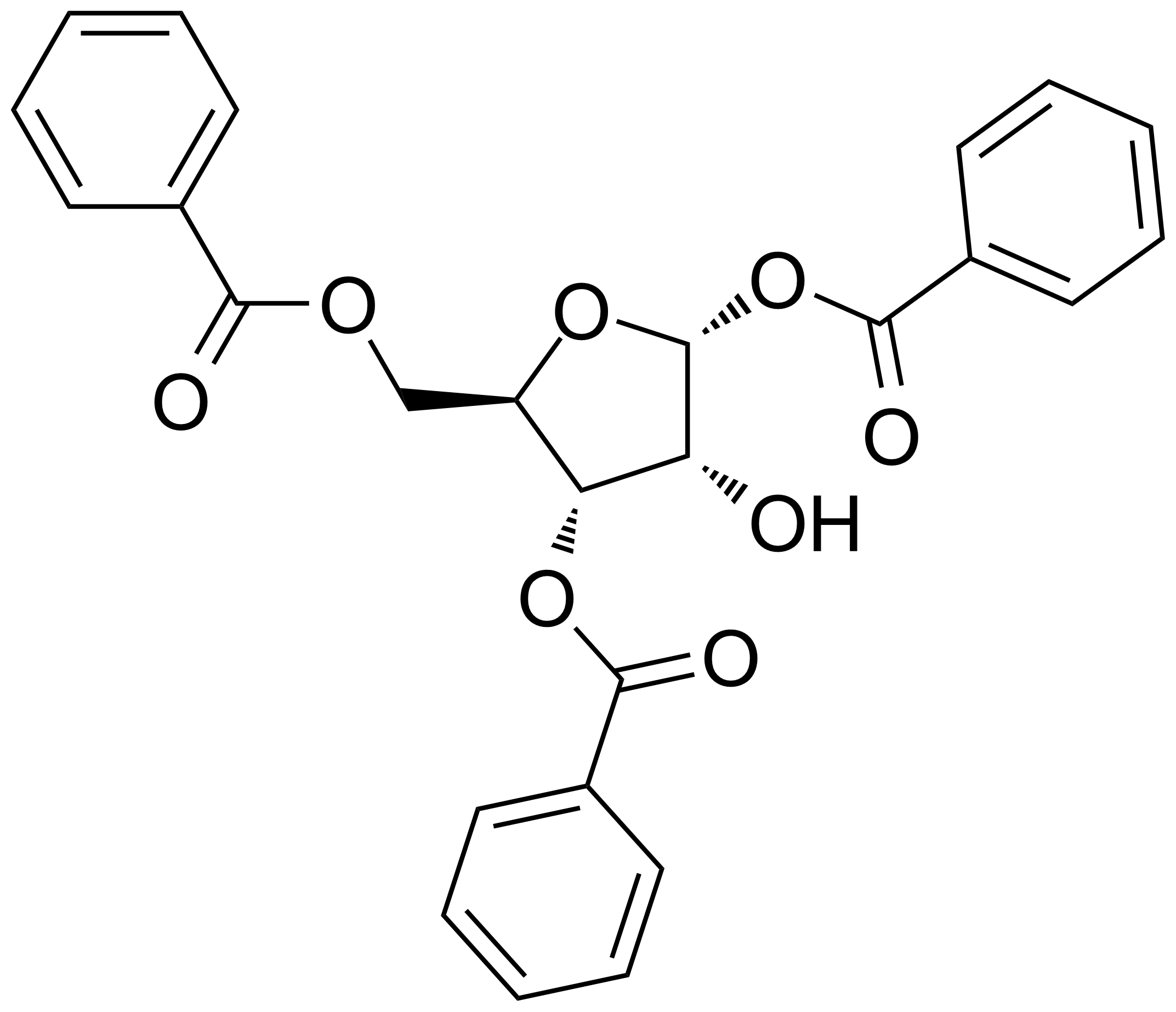 | [22224-41-5] | GEO-02210 |
| D-(+)-Ribonic-g-lactone | 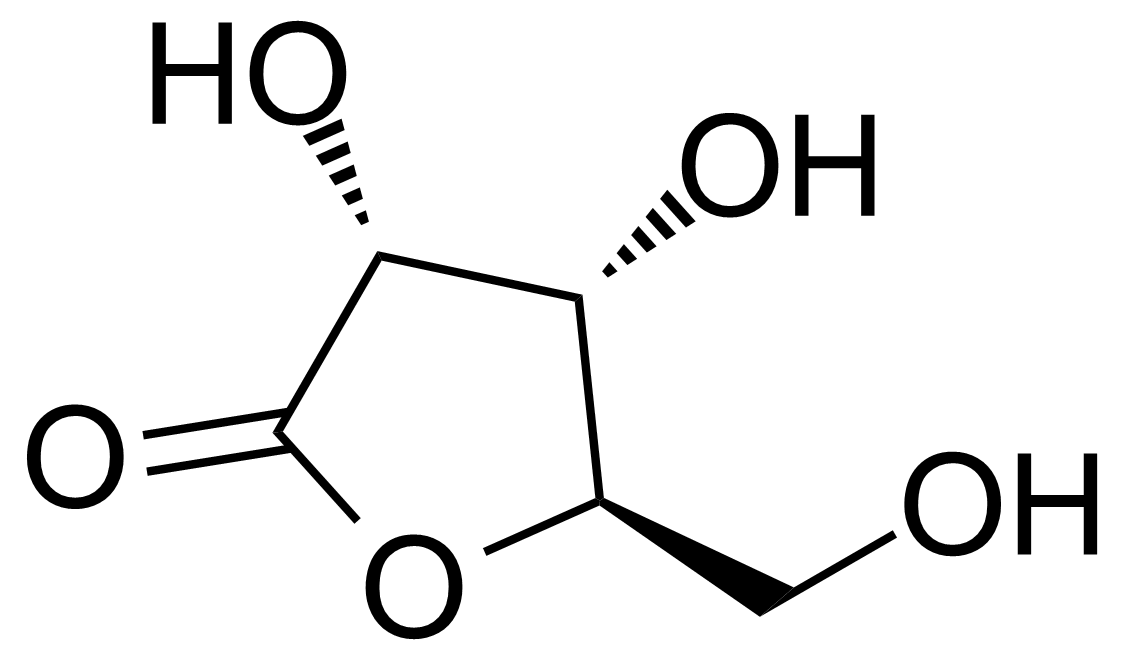 | [5336-08-3] | GEO-02211 |
| 2,3,4,6-Tetra-O-acetyl-b-D-glucopyranosyl isothiocyanate | 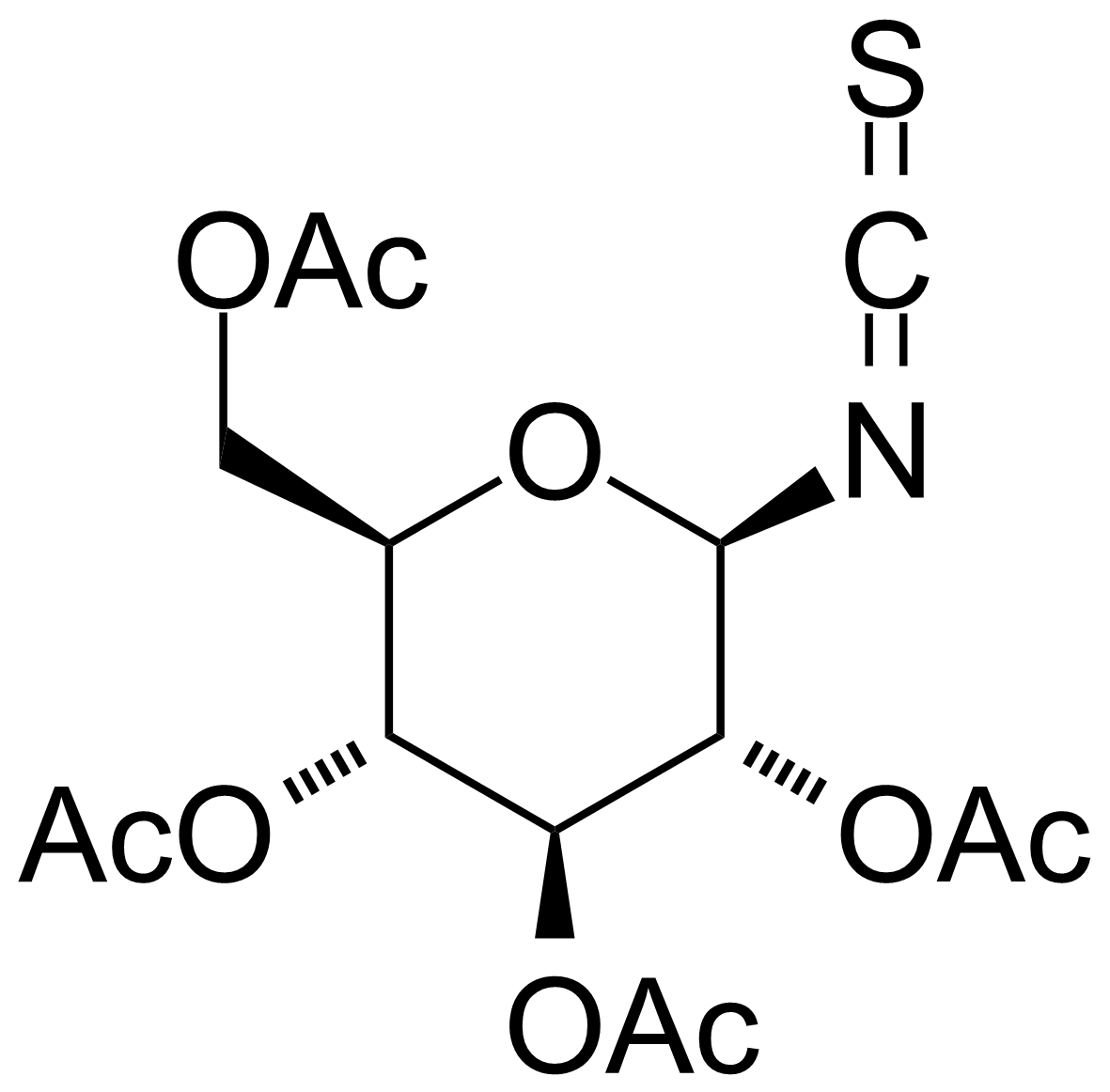 | [14152-97-7] | GEO-02227 |
| 2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl trichloroacetimidate | 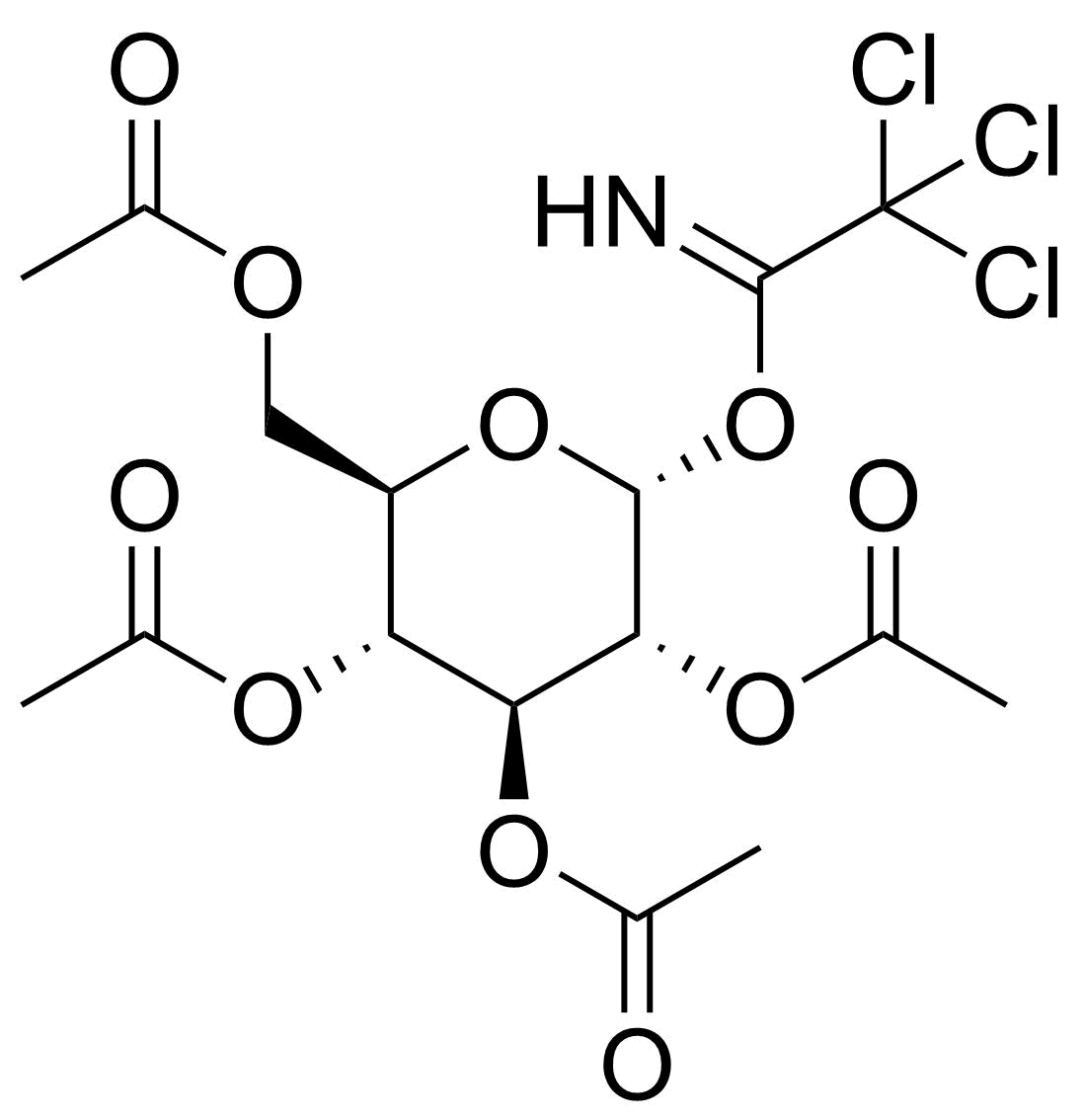 | [74808-10-9] | GEO-04520 |
| 2,3,4,6-Tetra-O-acetyl-beta-D-glucose | 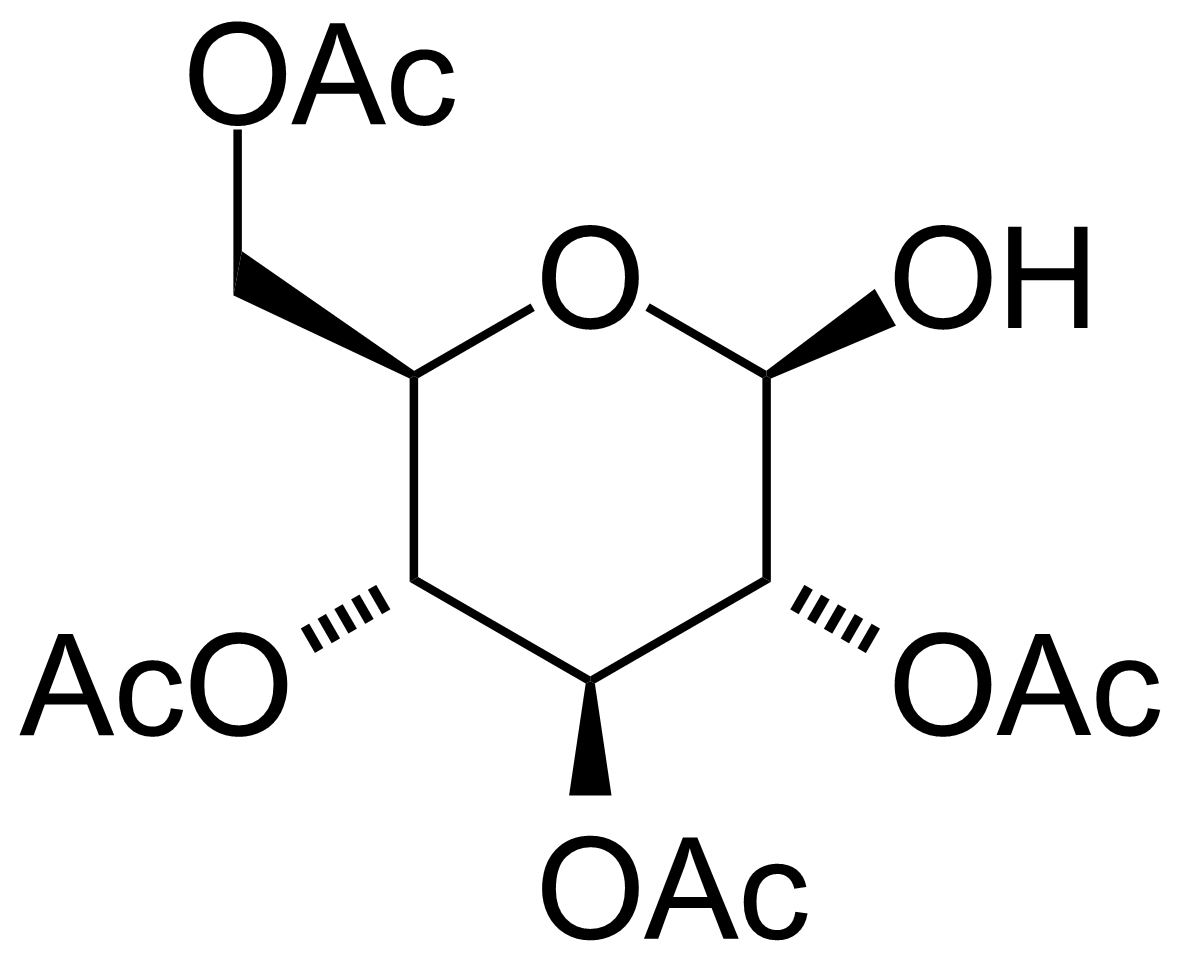 | [40437-08-9] | GEO-02226 |
| 2,3,4,6-Tetra-O-benzoyl-b-D-glucopyranosyl isothiocyanate | 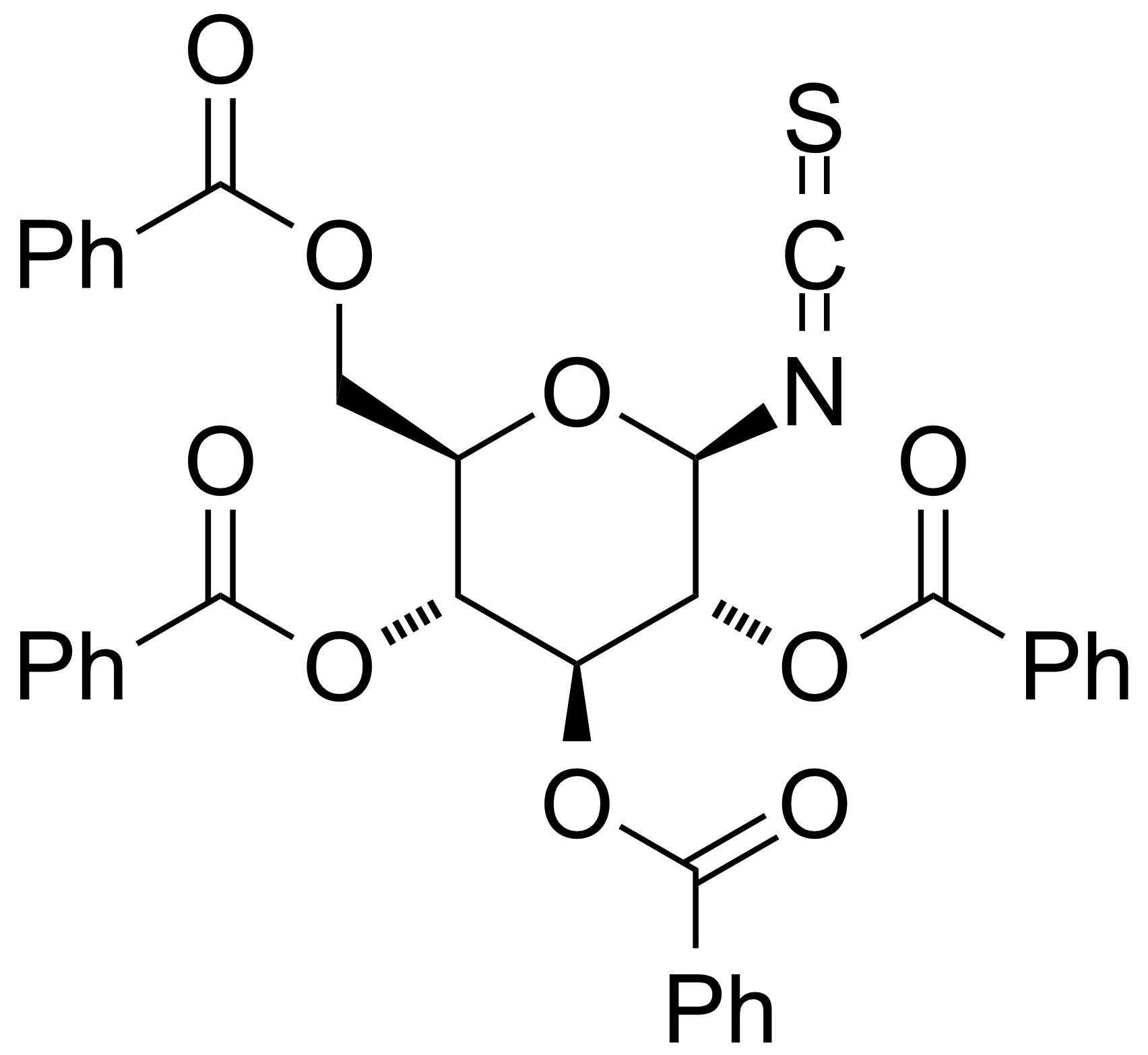 | [132413-50-4] | GEO-02230 |
| 1-Thio-beta-D-glucose tetraacetate | 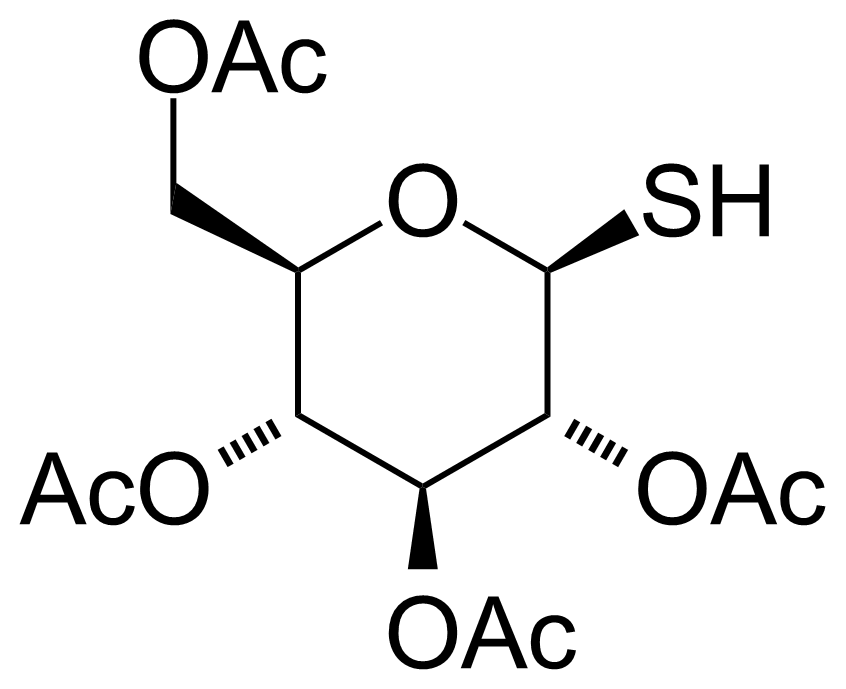 | [19879-84-6] | GEO-02308 |
| 2-(Trimethylsilylmethyl)allyl acetate | 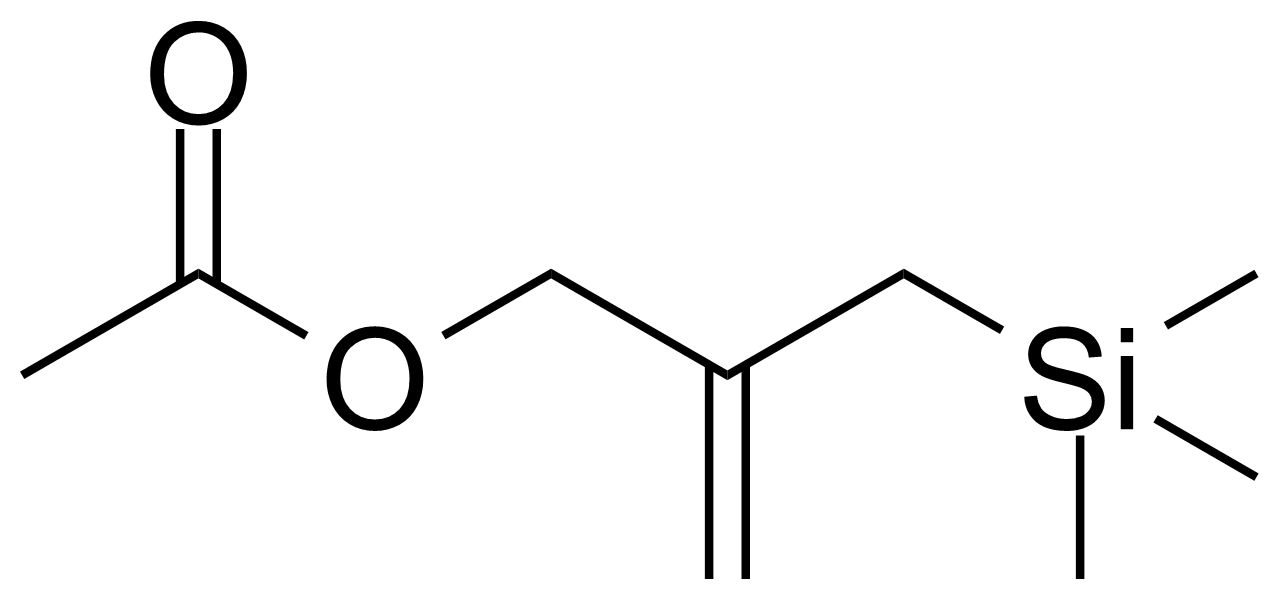 | [72047-94-0] | GEO-02621 |














![Structure of Benzo[b]thiophene-2-carboxylic acid methyl ester](https://georganics.sk/wp-content/uploads/2021/05/GEO-00287_Benzobthiophene-2-carboxylic_acid_methyl_ester.png)















![Structure of Diethyl 2-[4-(benzyloxy)benzylidene]malonate](https://georganics.sk/wp-content/uploads/2021/06/GEO-01038_Diethyl_2-4-benzyloxybenzylidenemalonate.png)








































![Structure of Dimethyl 2',6,6'-trimethyl-1',4'-dihydro-[3,4'-bipyridine]-3',5'-dicarboxylate](https://georganics.sk/wp-content/uploads/2021/06/GEO-03624_Dimethyl_266-trimethyl-14-dihydro-34-bipyridine-35-dicarboxylate.png)









































































![Structure of Methyl 4-[(chlorosulfonyl)methyl]benzoate](https://georganics.sk/wp-content/uploads/2021/05/GEO-03044_Methyl_4-chlorosulfonylmethylbenzoate.png)


















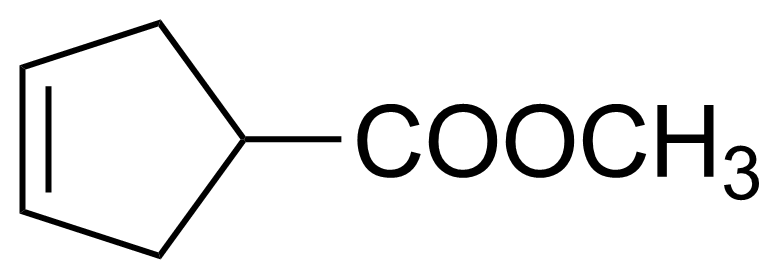










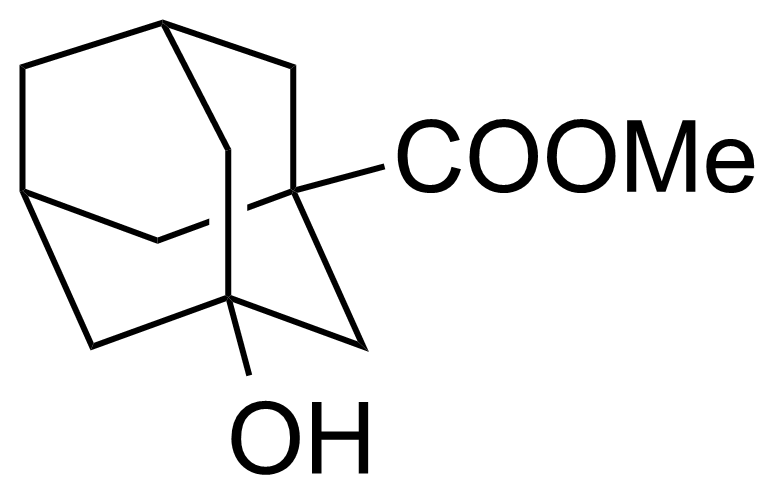






![Structure of Methyl 4-[(4-methoxycarbonylphenyl)carbamoylamino]benzoate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04233_Methyl_4-4-methoxycarbonylphenylcarbamoylaminobenzoate.png)

![Structure of Methyl 6-methylbenzo[d]thiazole-2-carboxylate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04028_Methyl_6-methylbenzodthiazole-2-carboxylate.png)
![Structure of Methyl 5-methylbenzo[d]thiazole-2-carboxylate](https://georganics.sk/wp-content/uploads/2021/05/GEO-04027_Methyl_5-methylbenzodthiazole-2-carboxylate.png)




![Structure of Methyl 2'-(5-nitrothiophen-2-yl)-1H,3'H-[2,5'-bibenzo[d]imidazole]-6-carboxylate](https://georganics.sk/wp-content/uploads/2021/06/GEO-01900_Methyl_2-5-nitrothiophen-2-yl-1H3H-25-bibenzodimidazole-6-carboxylate.png)


















