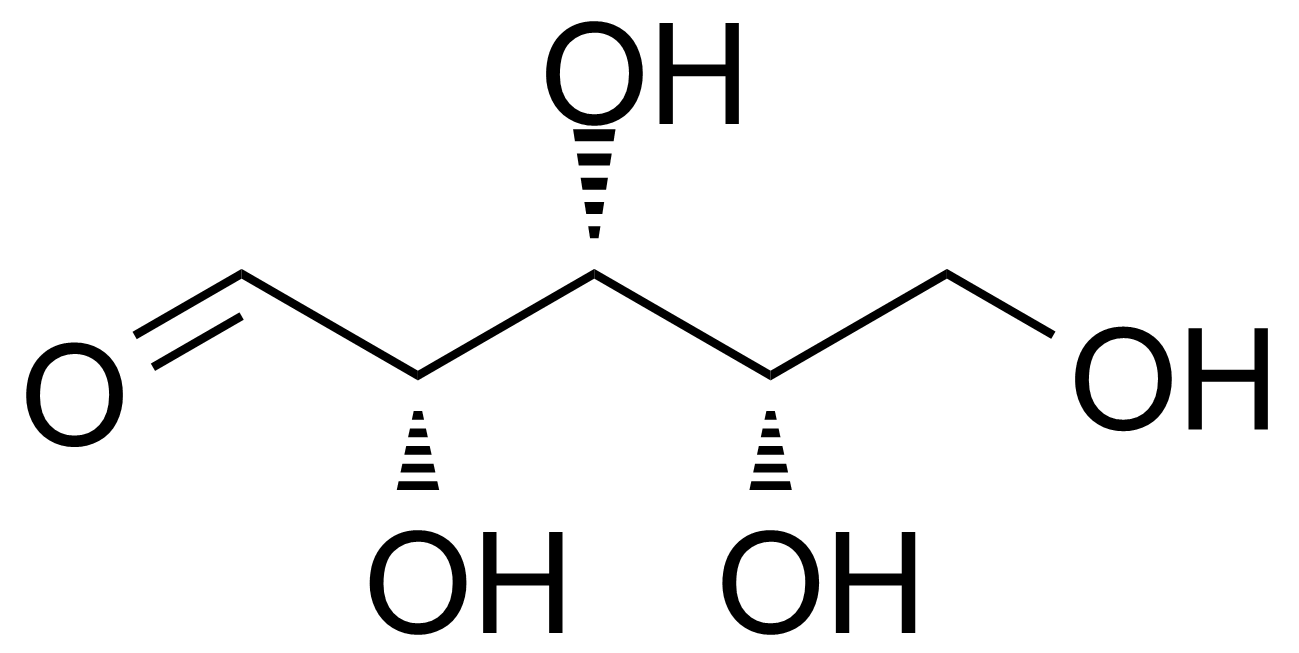
L-Xylose
CAS#[609-06-3]
G-codeGEO-02456
EC number210-174-1
Molecular formulaC5H10O5
Molecular weight150,13
Synonyms
L-(-)-xylose ; (2S,3S,4S)-oxane-2,3,4,5-tetrol ; L-Xylopyranose
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Product categorization
Special offer :
Main category
Second level
Description
L-Xylose is a useful chemical compound with a variety of research applications. We are pleased to offer high quality L-Xylose in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
L-xylose is used in organic synthesis as a chiral building block. It was used for the synthesis of L-ascorbic acid….
Show full descriptionL-Xylose [609-06-3] is a rare monosaccharide of the aldopentose type first isolated from wood, and named for it (ancient greek: ξύλον, xylon, "wood"). It is a white crystalline solid with the melting point of 144-145 °C.[1]
L-Xylose can be prepared by chemical route from D-gluconolactone [2] or from D-sorbitol.[3],[4] Enzymatic isomerization of the ketosugar L-xylulose to L-xylose has been presented as an alternative for low yields chemical synthesis. The starting material, L-xylulose can be produced by oxidation of the relatively cheap polyol, xylitol, using natural bacterial isolates as whole cell catalysts.[5]
Application of Furoin:
L-xylose is used in organic synthesis as a chiral building block. It was used for the synthesis of L-ascorbic acid.[6] Novel L-xylose derivatives have recently been reported to act as inhibitors of urinary glucose reabsorption, which suggests that they may find use in diabetes treatment.[7] Polyhydroxypyrrolidines derived from l-xylose have shown antitumor and anti-HIV properties and act as potent α- and β-glucosidase inhibitors, which also is of relevance for the development of diabetes drugs.[8]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________[1] T. G. Bonner, E. J. Bourne, S. E. Harwood, D. Lewis J. Chem. Soc. 1965, 121. doi:10.1039/JR9650000121
[2] W. B. Yang, S. S. Patil, C. H. Tsai, C. H. Lin, J. M. Fang Tetrahedron 2002, 58 (2), 253. doi:10.1016/S0040-4020(01)01146-2
[3] E. Dimant, M. Banay J. Org. Chem. 1960, 25 (3), 475. doi:10.1021/jo01073a621
[4] R. C. Hockett Production of l-xylose 1952, HEINZ M WUEST, US2584129A.
[5] A. Usvalampi, O. Turunen, J. Valjakka, O. Pastinen, M. Leisola, A. Nyyssölä Enzyme. Microb. Technol. 2012, 50 (1), 71. doi:10.1016/j.enzmictec.2011.09.009
[6] L. L. Salomon, J. J. Burns, C. G. King J. Am. Chem. Soc. 1952, 74 (20), 5161. doi:10.1021/ja01140a051
[7] N. C. Goodwin, R. Mabon, B. A. Harrison, M. K. Shadoan, Z. Y. Almstead, Y. Xie, J. Healy, L. M. Buhring, C. M. DaCosta, J. Bardenhagen, F. Mseeh, Q. Liu, A. Nouraldeen, A. G. E. Wilson, S. D. Kimball, D. R. Powell, D. B. Rawlins J. Med. Chem. 2009, 52 (20), 6201. doi:10.1021/jm900951n
[8] J. B. Behr, G. Guillerm Tetrahedron Lett. 2007, 48 (13), 2369. doi:10.1016/j.tetlet.2007.01.125
Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 1-Acenaphthenol | 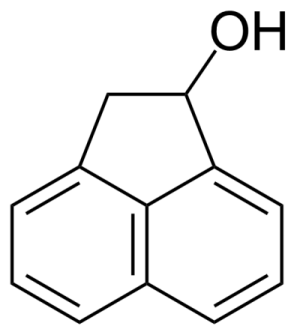 | [6306-07-6] | GEO-00001 | |
| 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 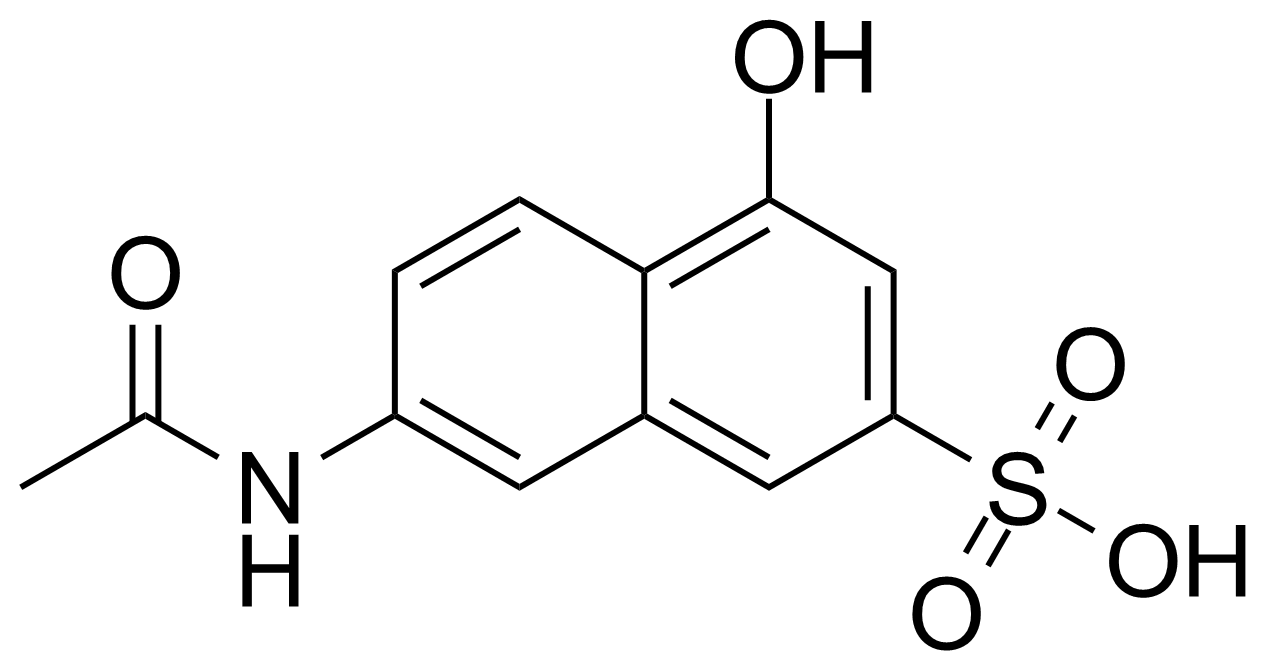 | [6334-97-0] | GEO-04013 | |
| 1-Adamantanemethanol | 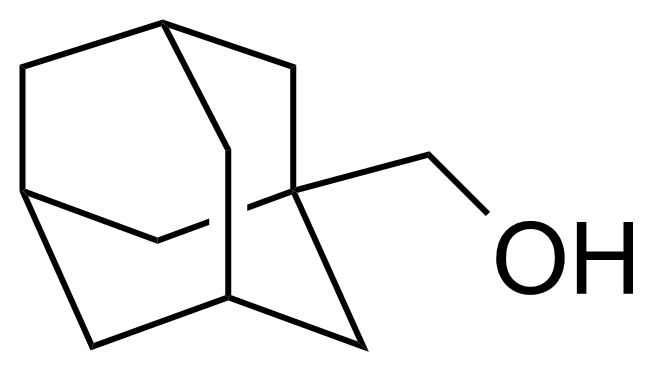 | [770-71-8] | GEO-04333 | |
| beta-D-Allopyranose | 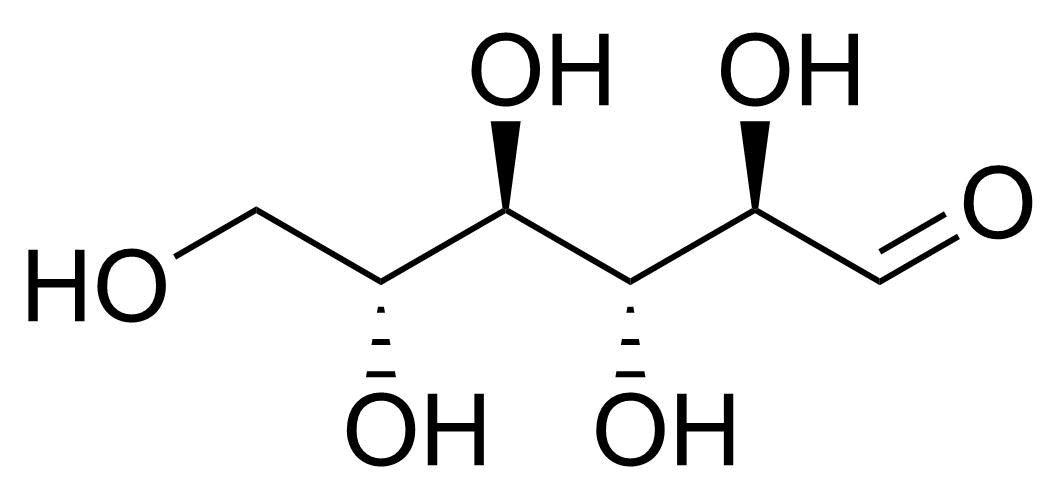 | [7283-09-2] | GEO-04660 | |
| D-Allose | 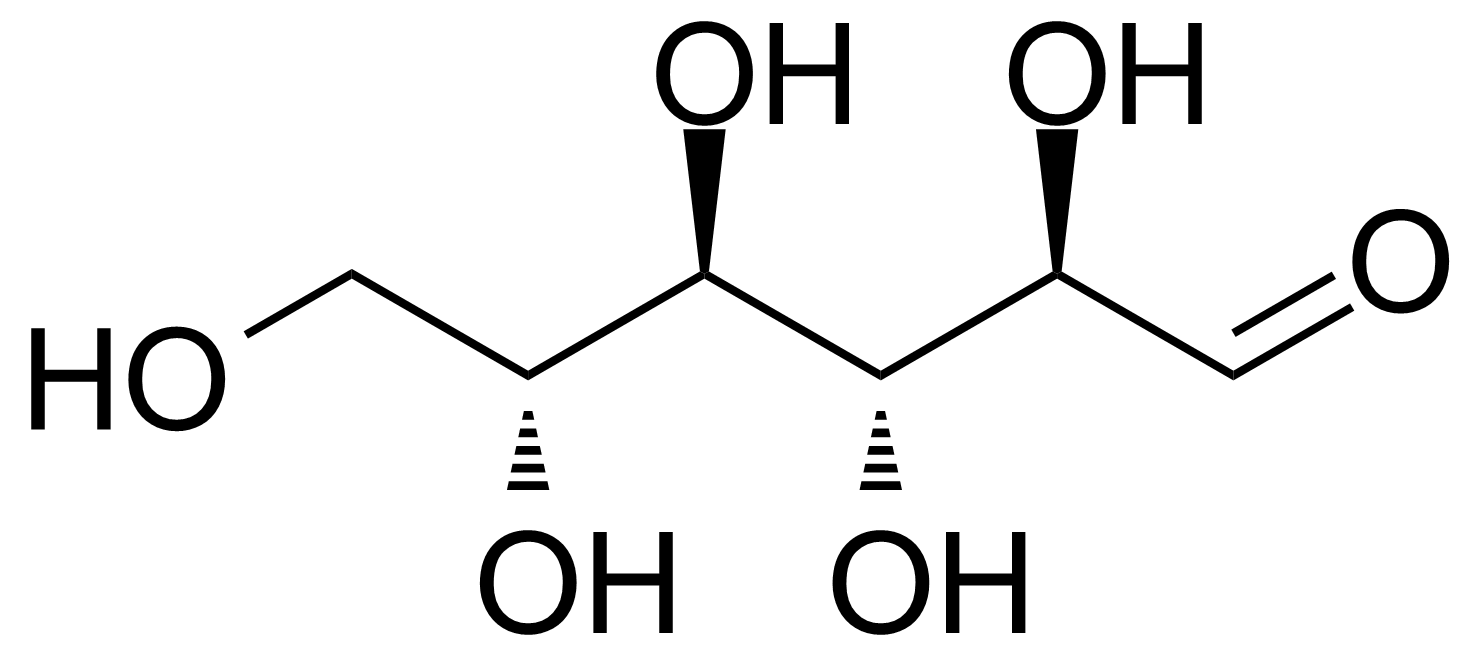 | [2595-97-3] | GEO-00057 | |
| L-Allose | 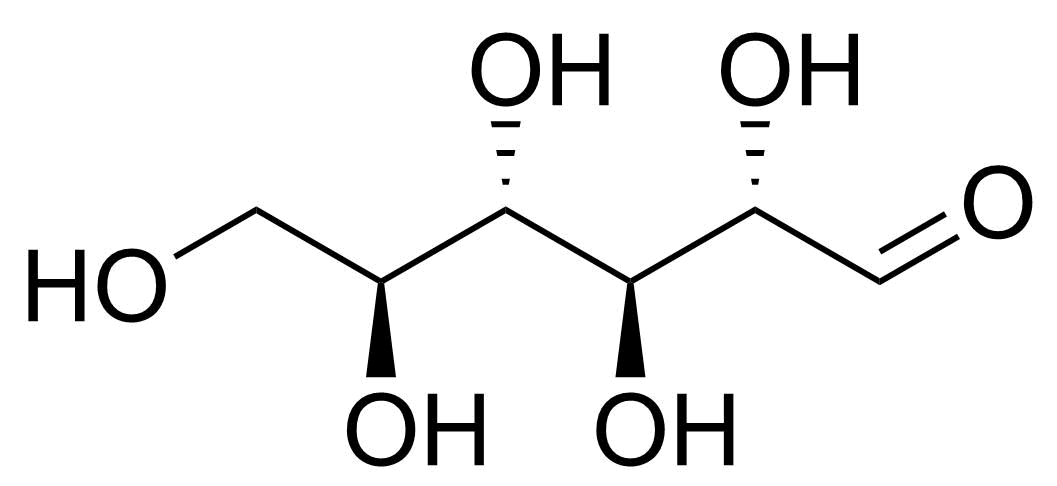 | [39392-62-6] | GEO-04661 | |
| 2-(Allyloxy)phenol | 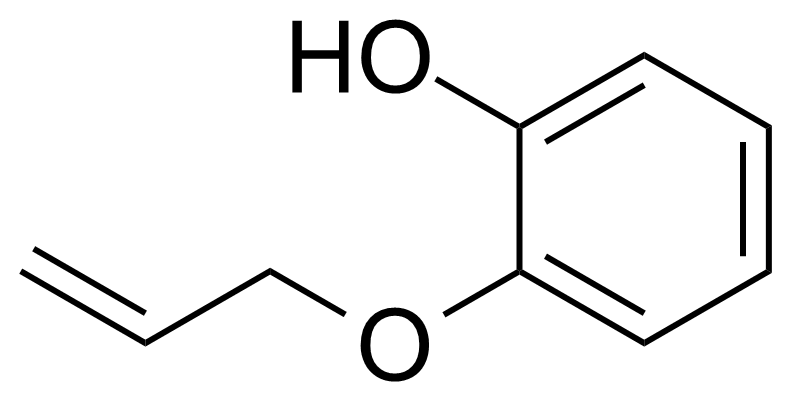 | [1126-20-1] | GEO-04471 | |
| D-Altrose | 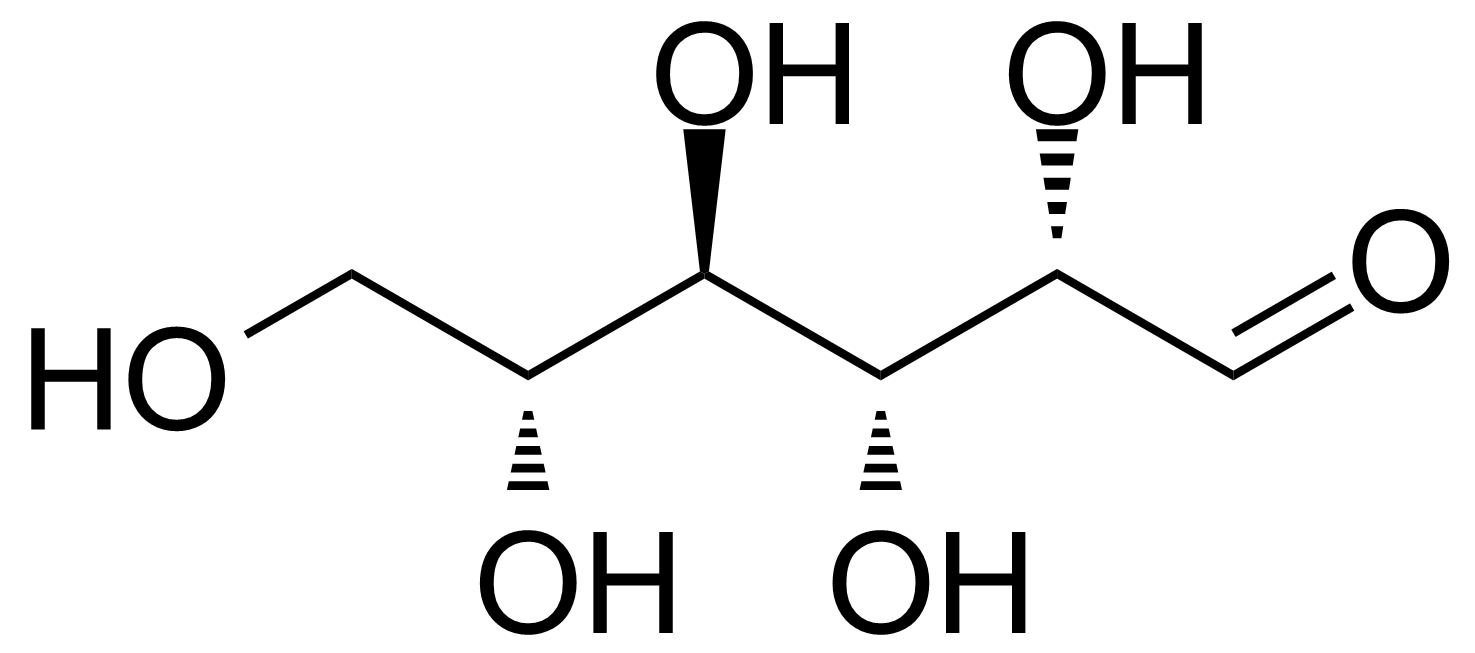 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 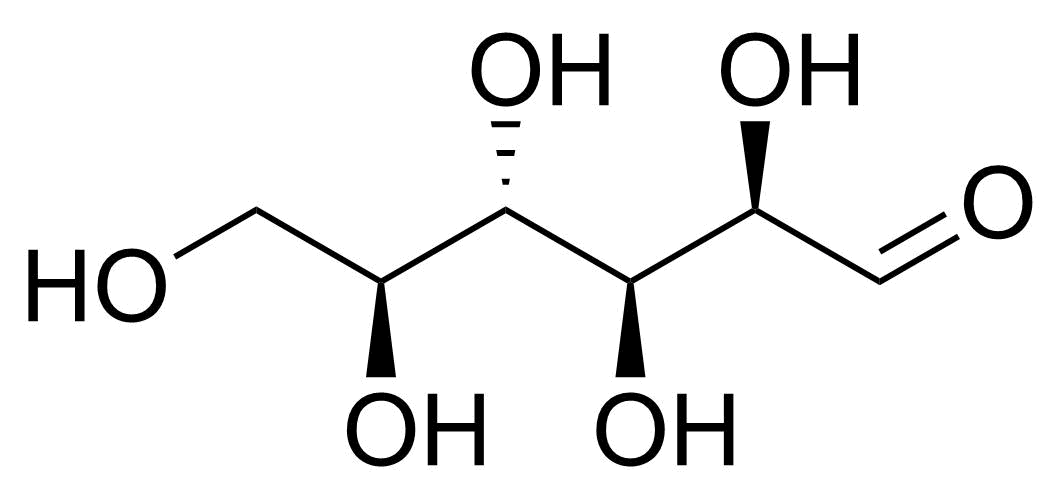 | [1949-88-8] | GEO-04662 | |
| (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 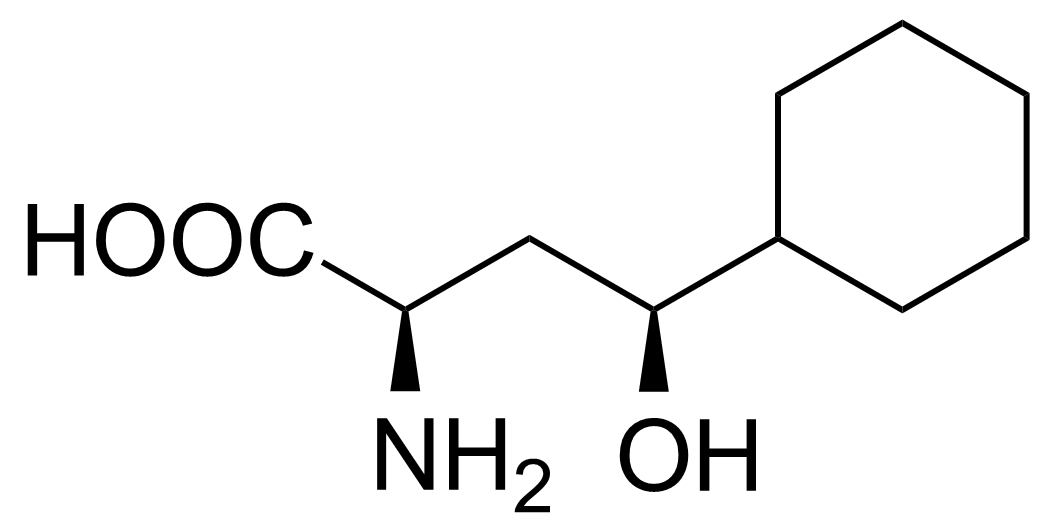 | [] | GEO-02717 |