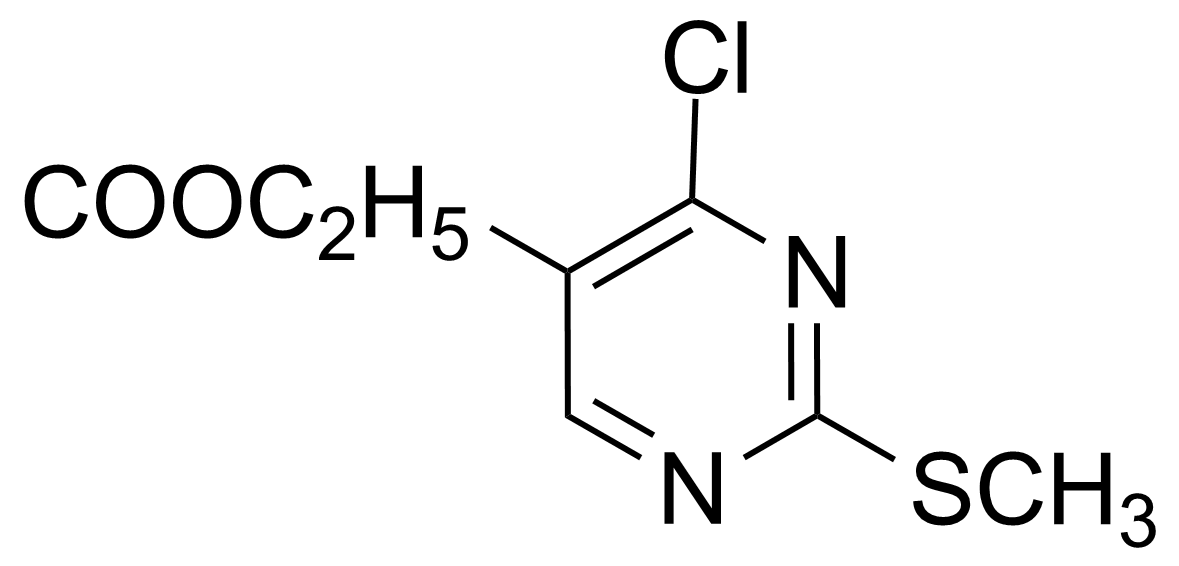
Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate
Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate ; Ethyl 4-chloro-2-methylthiopyrimidine-5-carboxylate ; Ethyl 4-chloro-2-(methylmercapto)pyrimidine-5-carboxylate ; 2-Methylthio-4-chloro-5-ethoxycarbonylpyrimidine ; 4-Chloro-5-carbethoxy-2-methylthiopyrimidine
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H315 – Causes skin irritation
H319 – Causes serious eye irritation
H335 – May cause respiratory irritation
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P302+352 – IF ON SKIN: Wash with soap and water
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Show full descriptionPreparation of Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate:
Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate can be prepared by a two step procedure. The condensation between S-methylisothiourea and diethyl ethoxymethylene malonate in basic conditions leads to the 4-oxopyrimidine sodium salt, which after treatment with phosphorous oxychloride under reflux affords desired 4-chloro derivative.[2]Application:
Pyrimidines have been used as suitable starting materials for the synthesis of novel scaffolds that are parent to DNA bases and derivatives, thus targeting compounds with relevant biological and pharmacological properties such as anticancer, anxiolytic, antioxidant, antiviral, antifungal, anticonvulsant, antidepressant, and antibacterial activities.[3] Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate was used in the synthesis of derivatives of pyrido[2,3-d]pyrimidin-7-one. These compounds are inhibitors of kinases such as Raf, including compounds that show anti-proliferative activity against cells, including against tumor cells, and are useful in the treatment of diseases including cancer.[4]Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-alpha-D-glucopyranosyl chloride | 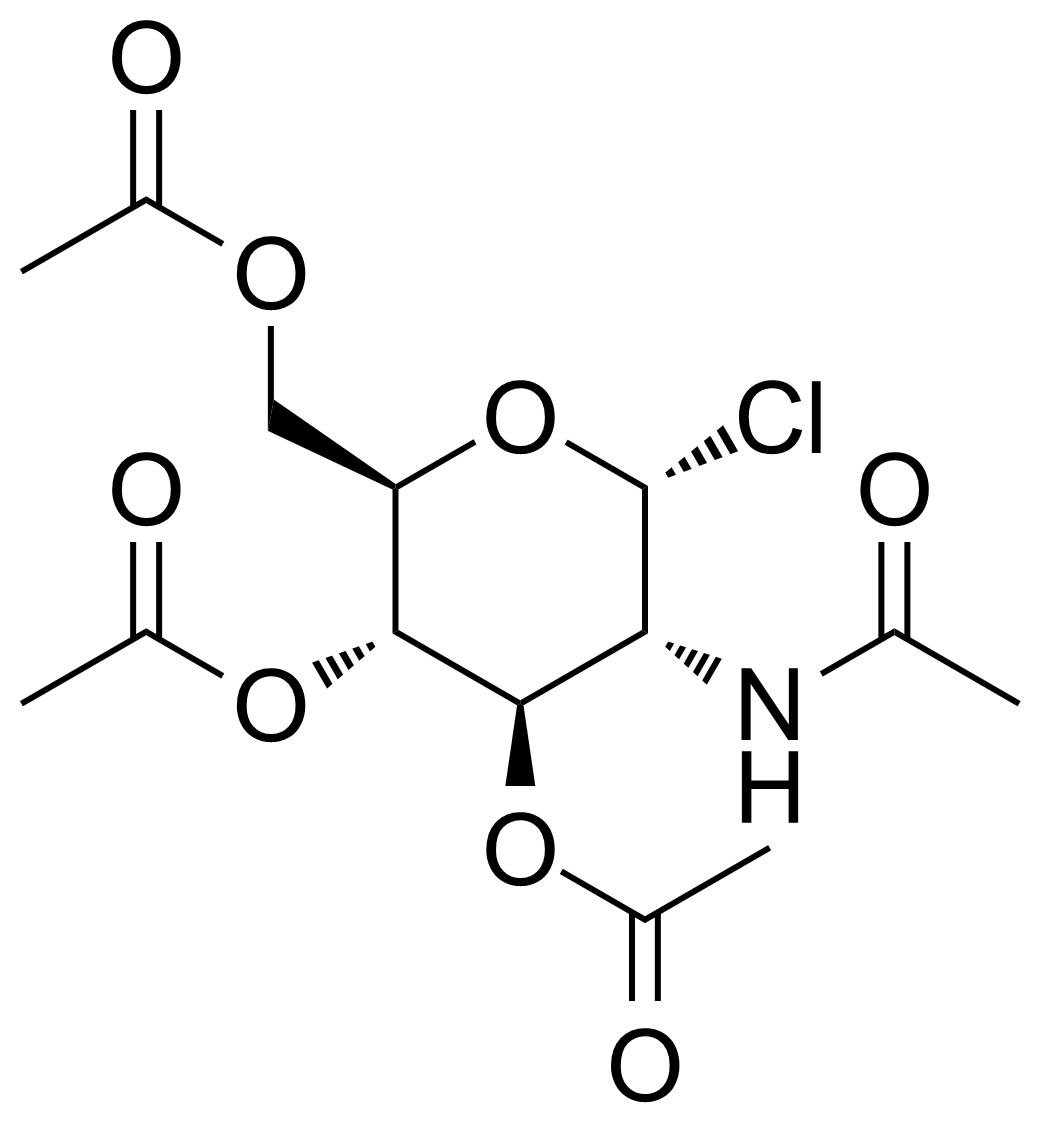 | [3068-34-6] | GEO-03374 | |
| Acetochloro-beta-D-glucose | 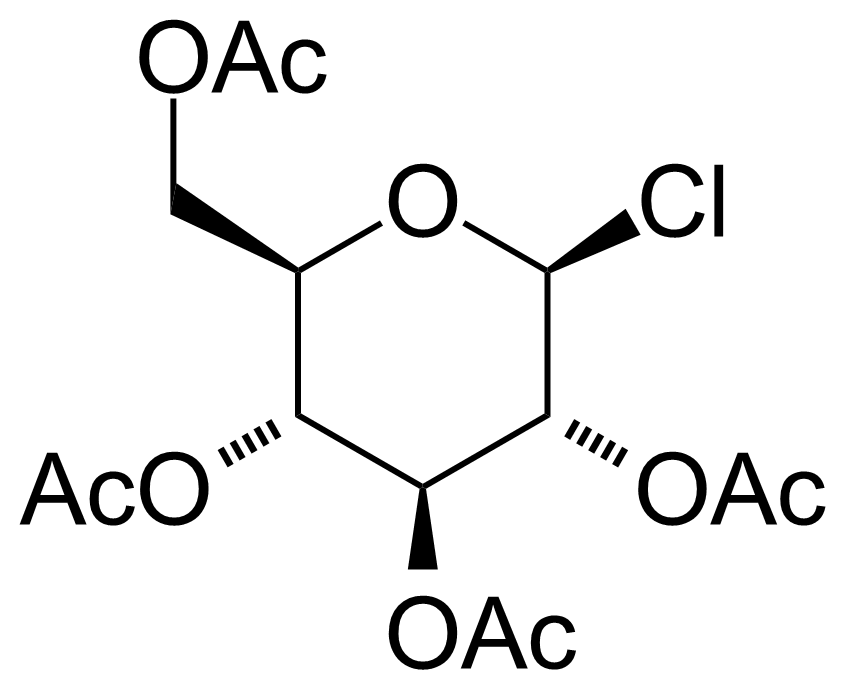 | [4451-36-9] | GEO-00008 | |
| 2-Acetyl-4-bromothiophene | 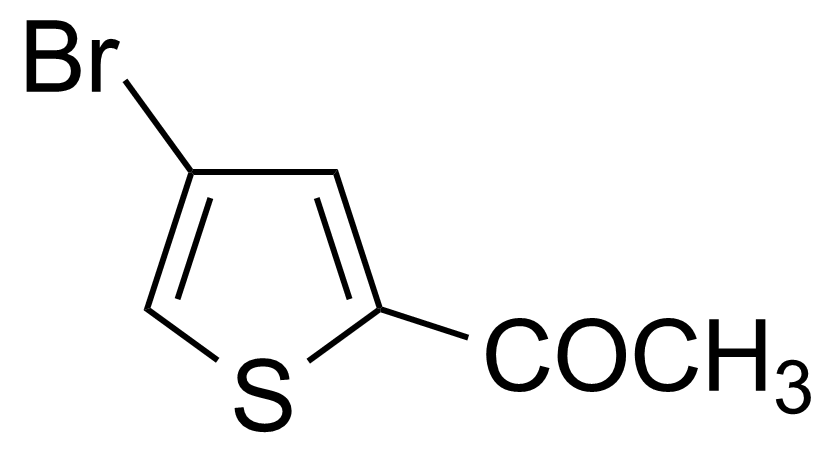 | [7209-11-2] | GEO-00023 | |
| 5-Amino-2-bromobenzoic acid | 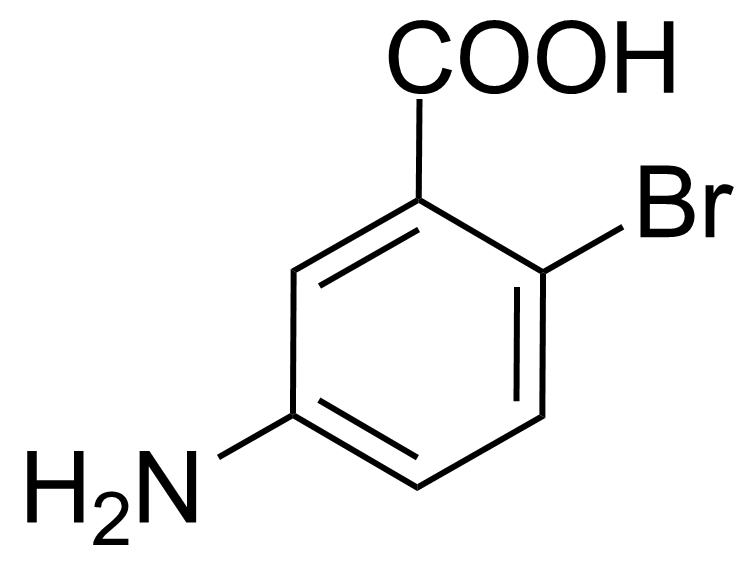 | [2840-02-0] | GEO-00082 | |
| 2-Amino-6-bromobenzothiazole | 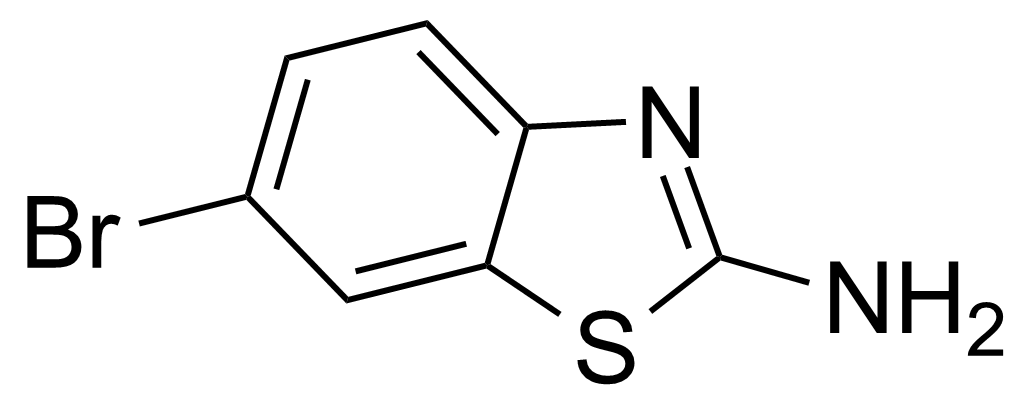 | [15864-32-1] | GEO-00083 | |
| 6-Amino-5-bromo-1-methyluracil monohydrate | 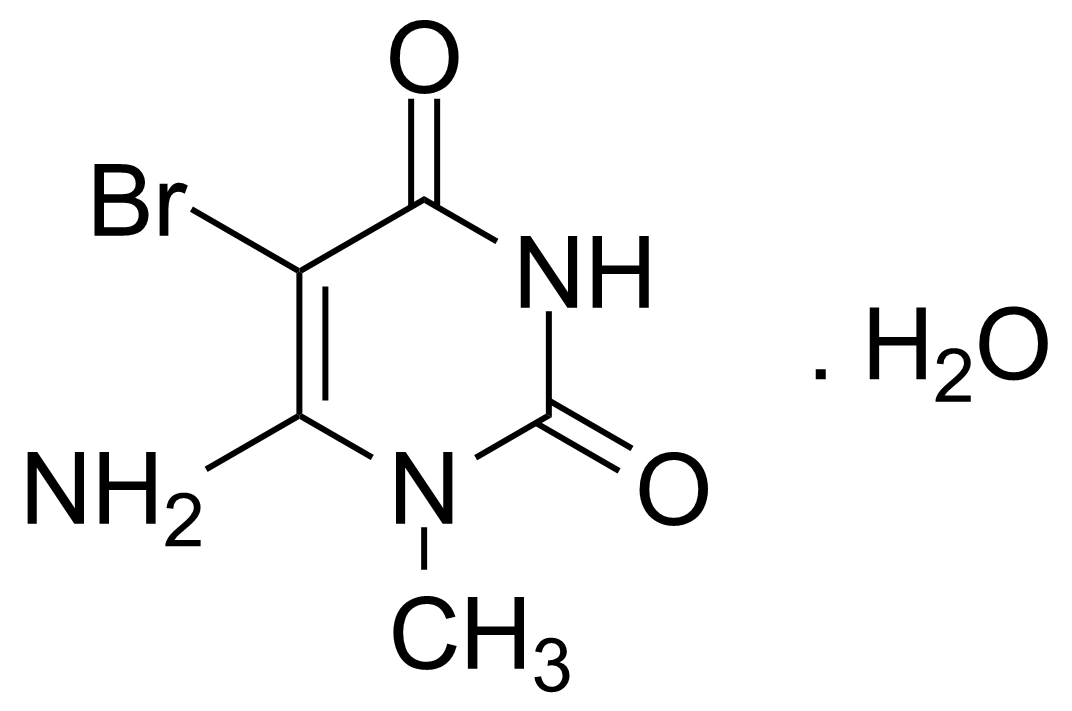 | [14094-37-2] | GEO-00087 | |
| 2-Amino-5-chlorobenzonitrile | 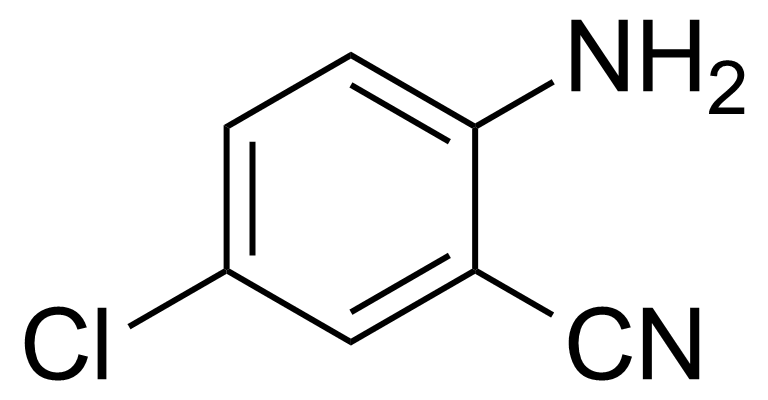 | [5922-60-1] | GEO-00097 | |
| 2-Amino-4-chlorobenzothiazole | 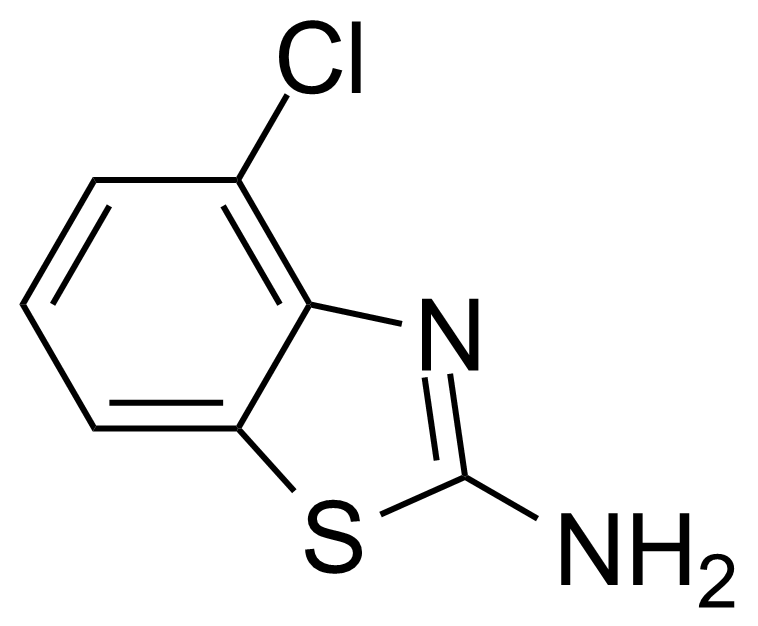 | [19952-47-7] | GEO-00099 | |
| 2-Amino-6-chlorobenzothiazole | 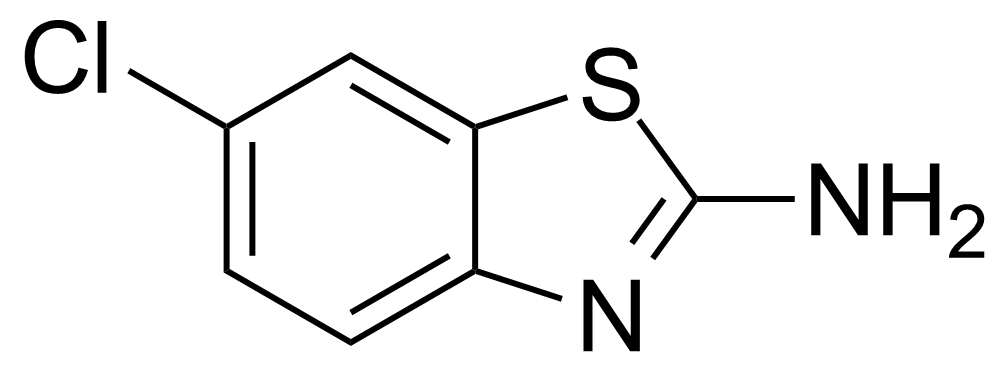 | [95-24-9] | GEO-02880 | |
| 2-Amino-4-chloro-3-iodopyridine | 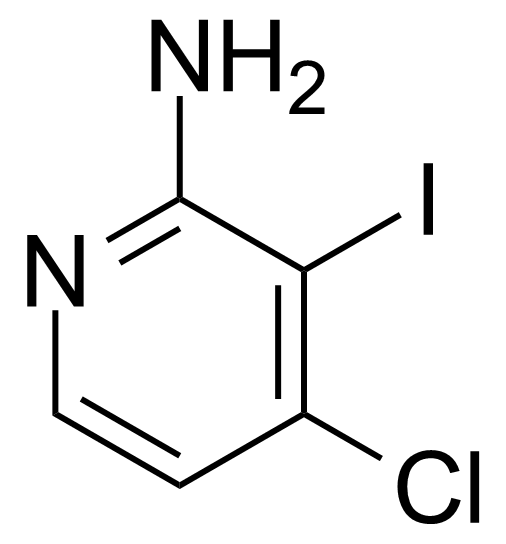 | [417721-69-8] | GEO-03750 |