 January 17, 2022
January 17, 2022Diethyl chlorophosphate – general description and application
General description of Diethyl chlorophosphate:
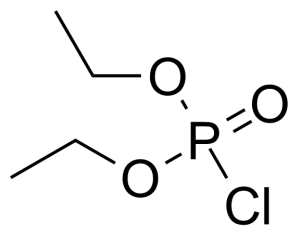
Diethyl chlorophosphate [814-49-3] or diethyl phosphorochloridate is a colorless to faith yellow clear liquid with the fruity odor and the boiling point of 60 °C/2 mmHg.[1] It acts as an cholinesterase inhibitor. It has high oral (LD50 = 11 mg/kg, rat) and very high dermal toxicity (LD50 = 8 μL/kg, rabbit), it is also toxic by inhalation.[2]
This compound is typically prepared by the chlorination of diethylphosphite with carbon tetrachloride, which is called Atherton–Todd reaction.[3] Another option of preparation is reaction of phosphoryl chloride with ethanol in the presence of triethylamine.[4]
Application of Diethyl chlorophosphate:
By using this substance, some ketones can be converted to enol phosphates which can be reduced to alkenes/alkanes or coupled with organometallic reagents to form substituted alkenes. Following enol phosphates can be then converted into β-keto phosphonates, useful for Horner-Emmons homologation.[5] It is also used in the synthesis of organophosphorus nerve agent mimics.[6] Phosphoroamidate linkages are found in a large array of biologically active natural products for example Microcin C7, Dinogunellin, Phosphoarginine, Phosphocreatine, Phosphoramidon, Phosmidosine and Agrocin.[7]
Product categorization (Chemical groups):
Main category:
______________________________________________________________________________________
[2] H. F. Smyth, Ch. P. Carpenter, C. S. Well, U. C. Pozzani, J. A. Striegel Am. Ind. Hyg. Assoc. J. 1962, 23, 95.
[3] G. M. Steinberg J. Org. Chem. 1950, 15, 637.
[4] G. Cahiez, O. Guerret, A. Moyeux, S. Dufour, N. Lefevre Org. Process Res. Dev. 2017, 21, 1542.
[5] J. R. Young e-EROS Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley & Sons, Ltd.,
[6] T. J. Dale, J. Rebek J. Am. Chem. Soc. 2006, 128, 4500.
[7] E. J. Itumoh, S. Data, E. M. Leitao Molecules 2020, 25, 3684.