 December 07, 2023
December 07, 2023D-Xylose – preparation and application
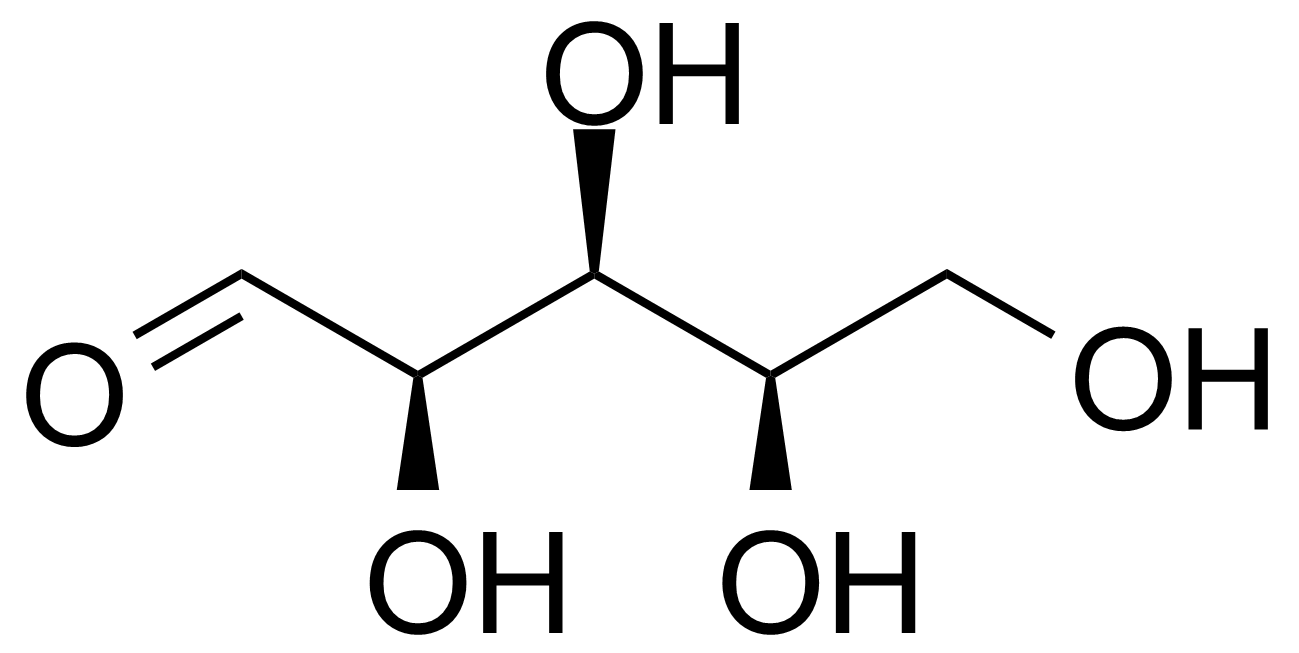
Xylose (cf. Ancient Greek: ξύλον, xylon, “wood”) is a sugar first isolated from wood and named for it. It is a reducing monosaccharide of the aldopentose type. D-xylose [58-86-6] is a white crystalline solid with the melting point of 143-145 °C.[1]
Preparation of D-Xylose:
Xylose is the main building block for the hemicellulose xylan, the third most abundant biopolymer on Earth. Xylose can be produced from cellulose and xylan containing materials (biomass) by first treating these extracts with an acid hydrolyzing agent in an aqueous medium to precipitate the lignins. The resulting aqueous solution is then passed through an ion exchange resin bed and the effluent from said bed which is dried is dissolved in methanol forming xylose as a crystalline material.[2]
Application:
Reduction of xylose by catalytic hydrogenation produces the sugar substitute xylitol.[3] The acid-catalysed degradation of hemicellulose gives furfural.[4] D-xylose absorption test is a medical test performed to diagnose conditions that present with malabsorption of the proximal small intestine due to defects in the integrity of the gastrointestinal mucosa.[5]
Product categorization (Chemical groups):
Main category:
Second level:
_______________________________________________________________________
[2] G. Jaffe, W. Szkrybalo, P. Weinert Process for producing xylose 1970, F Hoffmann La Roche AG, US3784408A
[3] S. Ur-Rehman, Z. Mushtaq, T. Zahoor, A. Jamil, M. A. Murtaza Food Sci. Nutr. 2015, 55 (11), 1514. doi:10.1080/10408398.2012.702288
[4] G. G. Millán, S. Hellsten, A. W. T. King, J. P. Pokki, J. Llorca, H. Sixta J. Ind. Eng. Chem 2019, 72, 354. doi:10.1016/j.jiec.2018.12.037
[5] R. M. Craig, A. J. Atkinson Jr. Gastroenterology 1988, 95 (1), 223. doi:10.1016/0016-5085(88)90318-6