 January 09, 2024
January 09, 2024Phenylboronic acid – preparation and application
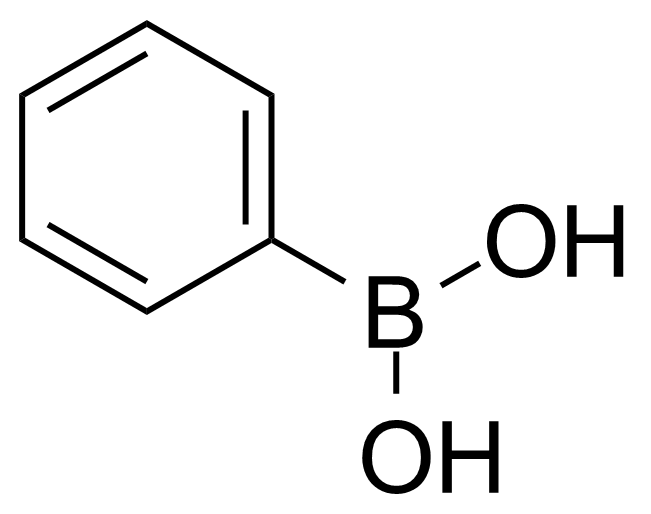
Phenylboronic acid (PBA) [98-80-6] or benzeneboronic acid is an organoboronic acid acid substituted with phenyl group. It is a white powder with the melting point of 215-216 °C.[1] Phenylboronic acid is soluble in most polar organic solvents. It is skin, eye and respiratory irritant which is harmful if swallowed (rat, oral, LD50 = 740 mg/kg).[2]
Preparation of Phenylboronic acid:
Benzeneboronic acid was first reported by Michaelis and Becker in 1880 who prepared benzeneboronyl dichloride by heating boron trichloride and diphenyl mercury at 180° to 200°C in a sealed tube. Benzeneboronyl dichloride was found to hydrolyze easily, giving benzeneboronic acid.[3] One of the most common preparations is based on reaction of phenylmagnesium bromide with trimethyl borate to form the ester, which is then hydrolyzed to the product.[4] Another synthetic approach, which gives lower yields, involves the reaction of phenyllithium with a borate ester.[5]
Application:
Phenylboronic acid participates in numerous cross coupling reactions. One of most famous example is the Suzuki–Miyaura reaction between boronic acid and organohalide in the presence of Pd(0) catalyst and base.[6] Heck-type cross coupling of phenylboronic acid and alkenes and alkynes has been demonstrated.[7] The thermal dehydration of boronic acids gives boroxines, the trimeric anhydrides of phenylboronic acid.3 The boronic acid-diol interaction has found many applications. Five and six membered cyclic arylboronate esters are formed upon binding between arylboronic acids and cis-1,2-diols or 1,3-diols respectively. Boronic acid-based sensors for sugars have been developed, particulary for the monitoring of glucose levels in blood of diabetic patients, and the development of glucose-responsive materials that automatically release insulin when glucose levels are too low.[8]
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
_______________________________________________________________________
[2] https://pubchem.ncbi.nlm.nih.gov/compound/66827#section=Toxicity
[3] A. Michaelis, P. Becker Chem. Ber. 1880, 13 (1), 58. doi:10.1002/cber.18800130118
[4] R. M. Washburn, E. Levens, C. F. Albright, F. A. Billig Org. Synth. 1959, 39, 3. doi:10.15227/orgsyn.039.0003
[5] R. L. Letsinger, I. Skoog J. Am. Chem. Soc. 1955, 77 (9), 2491. https://doi.org/10.1021/ja01614a039
[6] N. Miyaura, A. Suzuki J. Chem. Soc., Chem. Commun., 1979, (19), 866. doi:10.1039/C39790000866
[7] M. Sakai, M. Ueda, N. Miyaura Angew. Chem. Int. Ed. 1998, 37 (23), 3279. doi:10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M
[8] J. S. Hansen, J. B. Christensen, J. F. Petersen, T. Hoeg-Jensen, J. C. Norrild Sens. Actuators B: Chem. 2012, 161 (1), 45. doi:10.1016/j.snb.2011.12.024