 Janvier 01, 1970
Janvier 01, 1970Propyl ether – preparation and application
Unfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
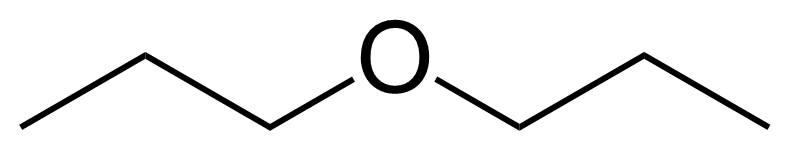
Propyl ether or 1-propoxypropane [111-43-3] is the symmetrical ether of two n-propyl groups. It is a colorless, flammable liquid with a sweet odor and the boiling point of 89-91 °C.[1] As is typical of ethers, dipropyl ether may slowly form explosive organic peroxides over long periods in storage. Antioxidants (such as butylated hydroxytoluene) are often added to ethers to prevent this process.
It can be prepared by by way of the Williamson ether synthesis in which n-propoxide anion is reacted with an n-propyl halide.[2] Dipropyl ether can be easily obtained from trimethylsilyl triflate or trimethylsilyl iodide catalyzed reductive coupling of propanal with triethylsilane.[3]
Application of Propyl ether:
As other ethers, it can be used as a solvent. It is also a versatile reagent for the construction of C-C bonds via sp3 α-C-H activation reaction.[4] It can be used in tert-butyl hydroperoxide (TBHP)-promoted tandem acylation/cyclization of 1,6-dienes under catalyst- and base-free conditions with a key step being cleavage of C(sp3)-H and C(sp3)-O bond of ether.[5] Propyl ether can be selectively oxidized at α-position to appropriate ester with sodium hypochlorite catalysed by ruthenium.[6]
Product categorization (Chemical groups):
Main category:
Second level:
_______________________________________________________________________
[2] P. Babiak, A. Němcová, L. Rulíšek, P. Beier J. Fluor. Chem. 2008, 129 (5), 397. doi:10.1016/j.jfluchem.2008.01.014
[3] M. B. Sassaman, K. D. Kotian, G. K. S. Prakash, G. A. Olah J. Org. Chem. 1987, 52 (19), 4314. doi:10.1021/jo00228a031
[4] S. Y. Zhang, F. M. Zhang, Y. Q. Tu Chem. Soc. Rev. 2011, 40, 1937. doi:10.1039/C0CS00063A
[5] X. J. Huang, F. H. Qin, Y. Liu, S. P. Wu, Q. Li, W. T. Wei Green Chem.2020, 22, 3952. doi:10.1039/D0GC00865F
[6] L. Gonsalvi, I. W. C. E. Arends, P. Moilanen, R. A. Sheldon Adv. Synth. Catal. 2003, 345 (12), 1321. doi:10.1002/adsc.200303124