 Januar 01, 1970
Januar 01, 1970Cyclopropylamine – general description and application
Unfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of Cyclopropylamine (CPA):
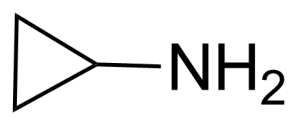
Cyclopropylamine (CPA) or aminocyclopropane [765-30-0] is a colorless liquid with the boiling point of 49-50 °C.[1] The density of a Cyclopropylamine is 0.82 g/cm3 (20 °C). Compound is harmful if swallowed (LD50 445 mg/Kg, rat, oral) and it can cause changes in structure or function of salivary glads, hypermotility or diarrhea.[2] It is highly flammable liquid and its vapour can cause severe skin burns and eye damage.
Cyclopropylamine is usually prepared from γ-butyrolactone in five steps sequence of ring-opening, esterification, cyclization, amidation and final Hoffman degradation of cyclopropanecarboxaminde.[3]
Application of Cyclopropylamine (CPA):
Cyclopropylamine is an important intermediate for synthesis of fluoroquinolone antibiotics such as Ciprofloxacin, that is recognized as one of the WHO’s Essential Medicines.[4] Many other fluoroquinolone derivatives (Gatifloxacin, Moxifloxacin, Gemifloxacin, Grepafloxacin, Garenoxacin, Sparfloxacin) contain cyclopropylamino moiety.[5] It is used in the preparation of insecticide Cyromazine, triazine insect growth regulator (it regulates the growth via inhibition of chitin synthesis).[6]
Product categorization (Chemical groups):
Main category:
Second level:
Hazard Identification:
H225 – Highly flammable liquid and vapour.
H302 – Harmful if swallowed.
H314 – Causes severe skin burns and eye damage.
P210 – Keep away from heat/sparks/open flames/hot surfaces. – No smoking.
P280 – Wear protective gloves/ protective clothing/ eye protection/ face protection.
P305 + P351 + P338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P310 – Immediately call a POISON CENTER or doctor/ physician
______________________________________________________________________________________
[2] National Technical Information Service., OTS0573763
[3]
P. Lipp, J. Buchkremer, H. Seeles, Justus Liebigs Ann. Chem. 1932, 1, 499.
T. Blackwell, H. L. Daughety, H. C. Grace, W. H. Oliver, Novartis AG, Process for the manufacture of cyclopropylamine, European Pattent Office, EP0205403A1, 4th June 1986.
[4] H. Lin, Ch. Dai, T. F. Jamison, K. F. Jensen, Angew. Chem., Int. Ed. 2017, 56, 8870.
[5] J. M. Paris 1.3 Chirality in Antibacterial Agents in Comprehensive Chirality 2012, Elsevier (ISBN 9780080951683).
[6] R.D. Ashford Ashford’s Dictionary of Industrial Chemicals 1994, London, England, Wavelength Publications Ltd. (ISBN: 9780952267409).