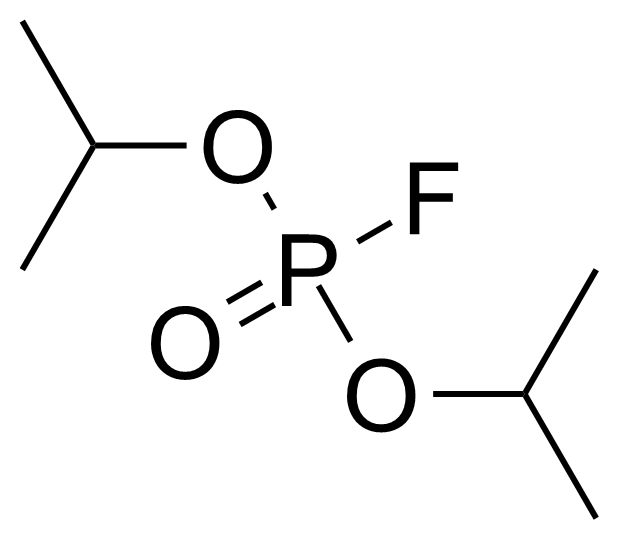
Diisopropyl fluorophosphate
DFP ; Diisopropyl phosphorofluoridate ; Phosphoric acid diisopropyl ester fluoride ; bis(propan-2-yl) fluorophosphonate ; Difluorophate ; Diflupyl ; Diisopropoxyphosphoryl fluoride ; Dyflos ; Floropryl ; Fluorodiisopropyl phosphate ; Fluostigmine ; Neoglaucit
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H300 – Fatal if swallowed
H310 – Fatal in contact with skin
H330 – Fatal if inhaled
P260 – Do not breathe dust/fume/gas/mist/vapours/spray:
P262 – Do not get in eyes, on skin, or on clothing:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P284 – Wear respiratory protection:
P302+P352+P310 – IF ON SKIN: Wash with plenty of water. Immediately call a POISON CENTER/ doctor.
P304+P340+P310 – IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/ doctor.
Product categorization
Description
Diisopropyl fluorophosphate is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Diisopropyl fluorophosphate in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Diisopropyl fluorophosphate is known to bind and inhibit serine proteases and other unidentified esterases…
Show full descriptionGeneral description of Diisopropyl fluorophosphate:
Diisopropyl fluorophosphate (DFP, DIFP, DIPF) [55-91-4] or isofluorophate or diisopropyl phosphorofluoridate or phosphoric acid diisopropyl ester fluoride is clear colorless or slightly yellow oily liquid with boiling point of 183 °C.[1] It is a a synthetic dialkyl phosphate with a weak fruity odor. Significant variability has been observed in toxicological studies using commercially available DFP (LD50 values range from 0.0027 mg/kg to 6.4 mg/kg in the mouse model.[2] It is highly toxic by skin absorption and inhalation. Diisopropyl fluorophosphate is usually made by reaction of isopropyl alcohol with phosphorus trichloride, forming diisopropylphosphite, which is chlorinated and further reacted with sodium fluoride to replace the chlorine atom with fluorine, thus giving diisopropyl fluorophosphate.Application of Diisopropyl fluorophosphate:
Isofluorophate is a powerful neurotoxin often used in research studies as a surrogate for organophosphorus nerve agents such as sarin (GB) and soman (GD) due to its ability to effectively inhibit the enzyme acetylcholinesterase.[3] It is typical example of organophosphorus insecticide, firstly patented by Monsanto chemical company in 1944.[4] DFP is known to bind and inhibit serine proteases and other unidentified esterases. DFP has been used and is still used as a tool for many toxicological and pharmacological applications in which the consequence of protease inhibition or cholinergic effects is needed to study a pharmacological or cell biology process.[5]Product categorization (Chemical groups):
Main category: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 2-Aminoethyl dihydrogen phosphate | 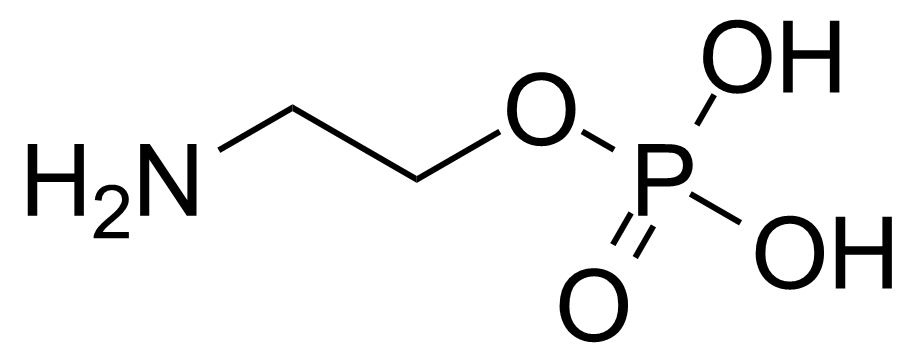 | [1071-23-4] | GEO-00129 | |
| 2-Aminoethyl dihydrogen phosphate magnesium salt (2:1) | 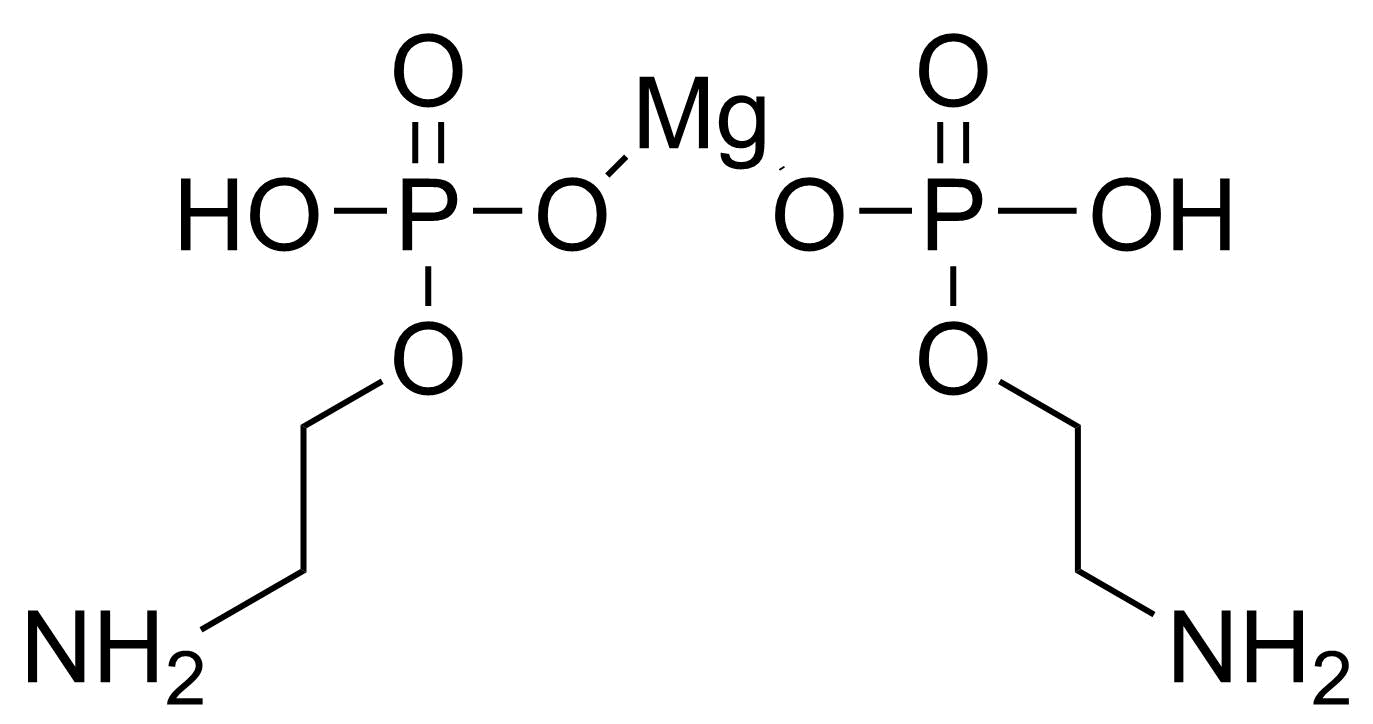 | N/A | GEO-04286 | |
| (Aminomethyl)phosphonic acid | 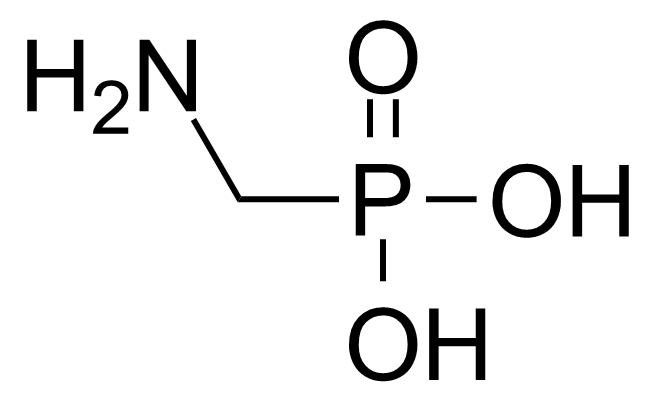 | [1066-51-9] | GEO-00169 | |
| (3-Aminopropyl)phosphonic acid | 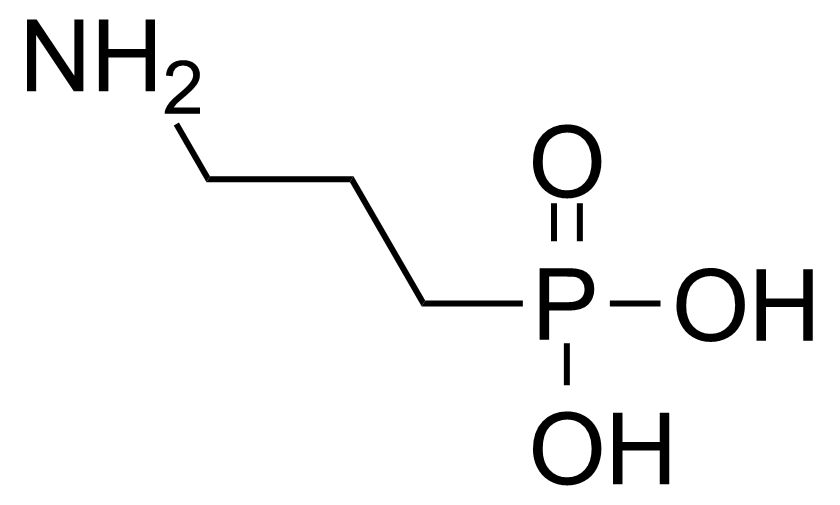 | [13138-33-5] | GEO-00218 | |
| Benzo[d]isoxazol-3-yl diphenyl phosphate | ![Structure of Benzo[d]isoxazol-3-yl diphenyl phosphate](https://georganics.sk/wp-content/uploads/2021/05/GEO-00266_Benzodisoxazol-3-yl_diphenyl_phosphate.png) | [94820-31-2] | GEO-00266 | |
| Benzyl dimethylphosphonoacetate | 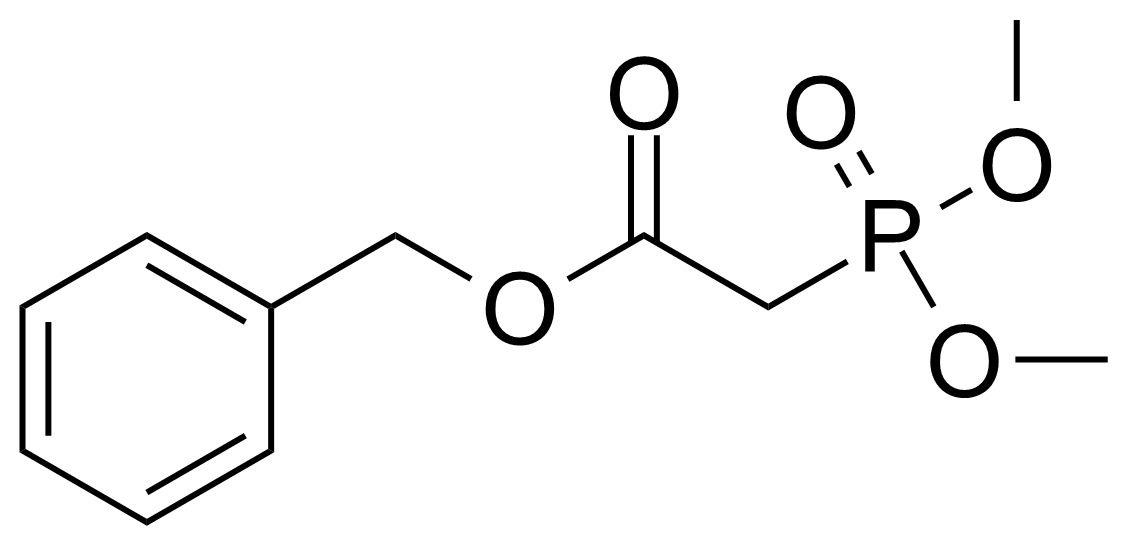 | [57443-18-2] | GEO-02882 | |
| Benzyl(triphenylphosphoranylidene)acetate | 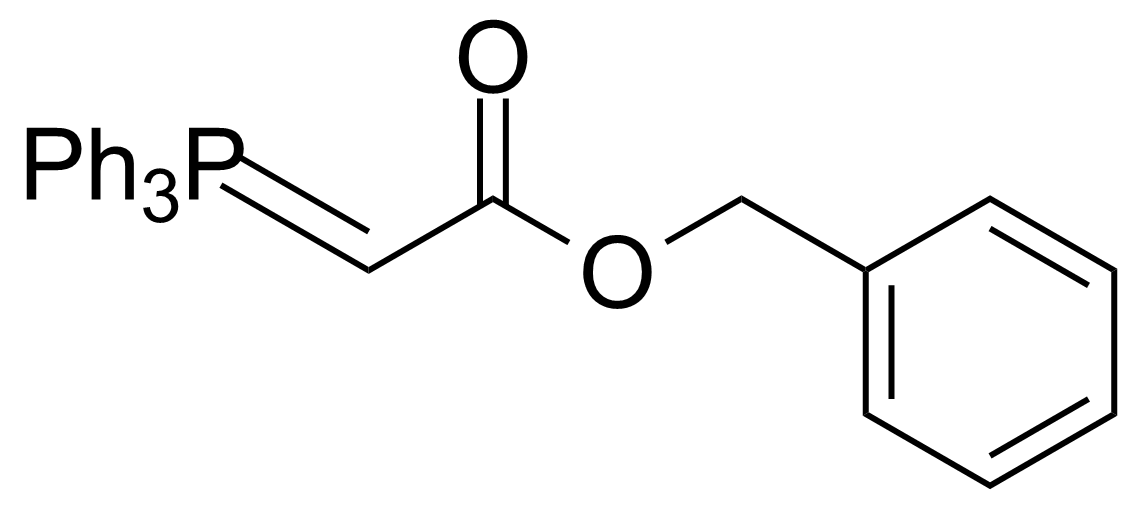 | [15097-38-8] | GEO-02511 | |
| Bis(dichlorophosphino)methane | 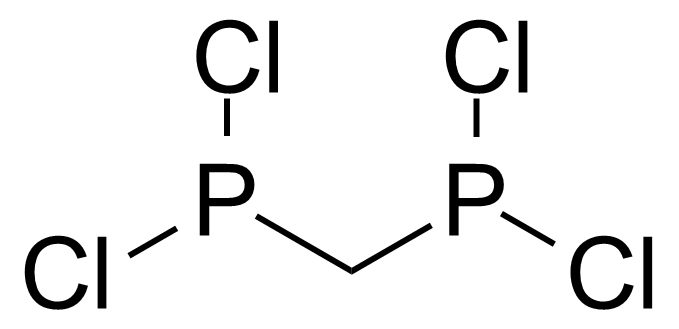 | [28240-68-8] | GEO-03420 | |
| Bis(diethylamino)chlorophosphine | 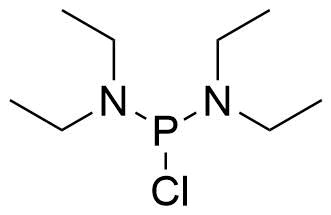 | [685-83-6] | GEO-04741 | |
| N,N-Bis(diphenylphosphino)amine | 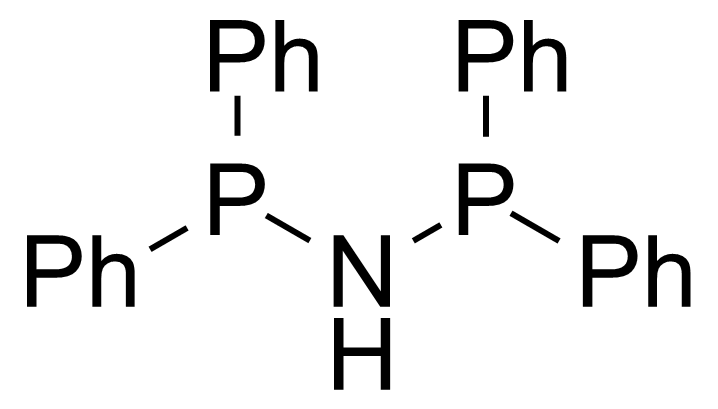 | [2960-37-4] | GEO-04010 |