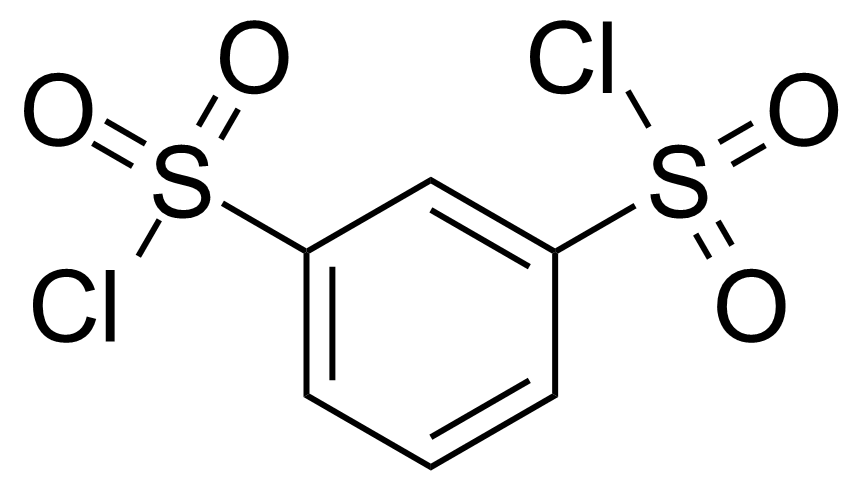
Benzene-1,3-disulfonyl chloride
1,3-Benzenedisulfonyl dichloride ; 3-Chlorosulfonylbenzenesulfonyl chloride ; m-Benzenedisulfonyl chloride ; m-Benzenedisulfonyl dichloride ; m-Phenylenedisulfonyl chloride ; 1,3-Benzenedisulfonyl chloride
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information
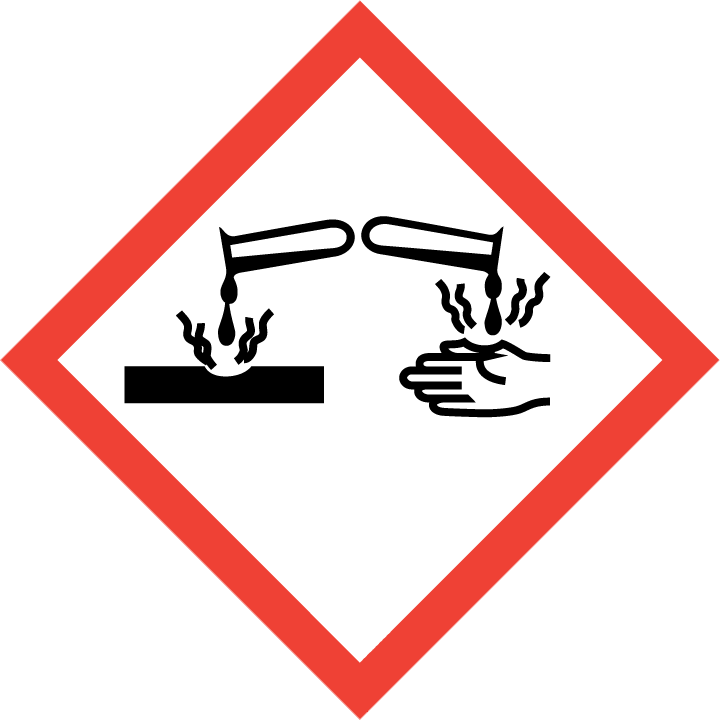

H314 – Causes severe skin burns and eye damage
H317 – May cause an allergic skin reaction
H334 – May cause allergy or asthma symptoms or breathing difficulties if inhaled
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
P310 – Immediately call a POISON CENTER or doctor/physician:
Product categorization
Description
Benzene-1,3-disulfonyl chloride is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Benzene-1,3-disulfonyl chloride in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
1,3-Benzenedisulfonyl chloride is useful reagent in organic chemistry for the preparation of pharmaceutical intermediates…
Show full descriptionGeneral description:
Benzene-1,3-disulfonyl chloride (BDD) [585-47-7] or 1,3-benzenedisulfonyl chloride is dichloride of 1,3-benzenedisulfonic acid. It is a colorless (white) crystalline solid with the melting point of 59-62 °C.[1] It is soluble in common organic solvents. Compound is sensitive to moisture and reacts in water therefore should be stored in dry conditions. It has corrosive properties and can cause severe skin burns and eye damage. Convenient laboratory preparation is based on chlorination of appropriate disodium salt using phosphorus chloride[1] or thionyl chloride[2]. Alternative method starts with 1,3-benzenedithiol, which reacts with chlorine in acetic acid.[3]Application of Benzene-1,3-disulfonyl chloride:
1,3-Benzenedisulfonyl chloride is useful reagent in organic chemistry for the preparation of pharmaceutical intermediates.[4] It was used in the synthesis of isonitriles as dehydration agent of formamides.[2] Benzene-1,3-disulfonyl chloride is commonly used in the synthesis of biological active sulfonamides[5] and in the preparation of composite polysulfonamide membranes.[6]Product categorization (Chemical groups):
Main category: Second level: Third level: ______________________________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 3-Acetylbenzene-1-sulfonyl chloride | 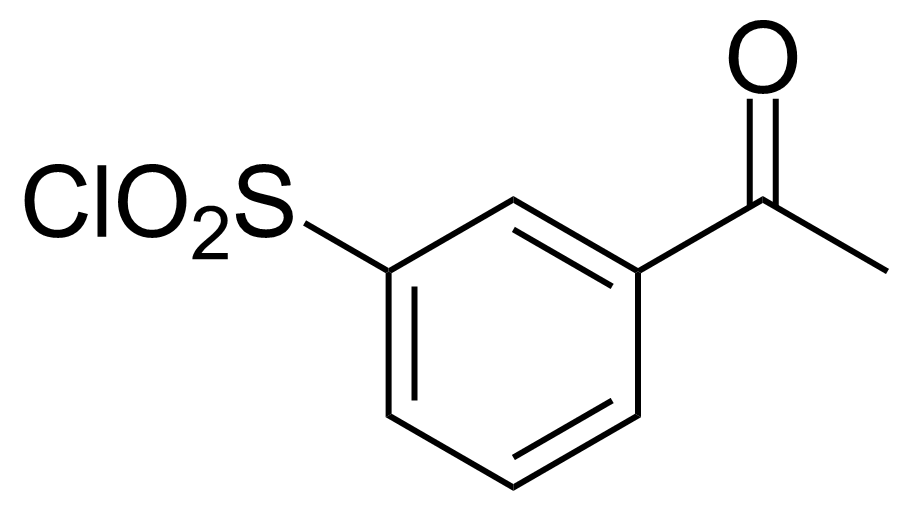 | [73035-16-2] | GEO-03453 | |
| 1-Acetyl-5-indolinesulfonoyl chloride |  | [52206-05-0] | GEO-02639 | |
| Benzene-1,3-disulfonyl chloride |  | [585-47-7] | GEO-03722 | |
| 1,3-Benzothiazole-6-sulfonyl chloride | 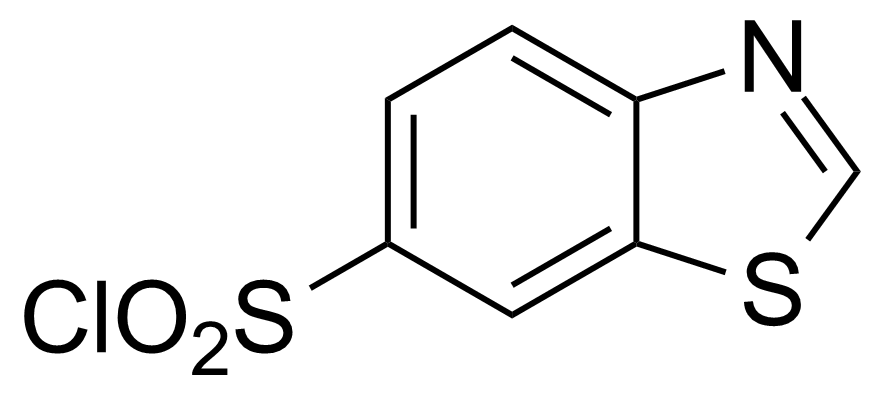 | [181124-40-3] | GEO-00282 | |
| 4-Benzyloxybenzenesulfonyl chloride | 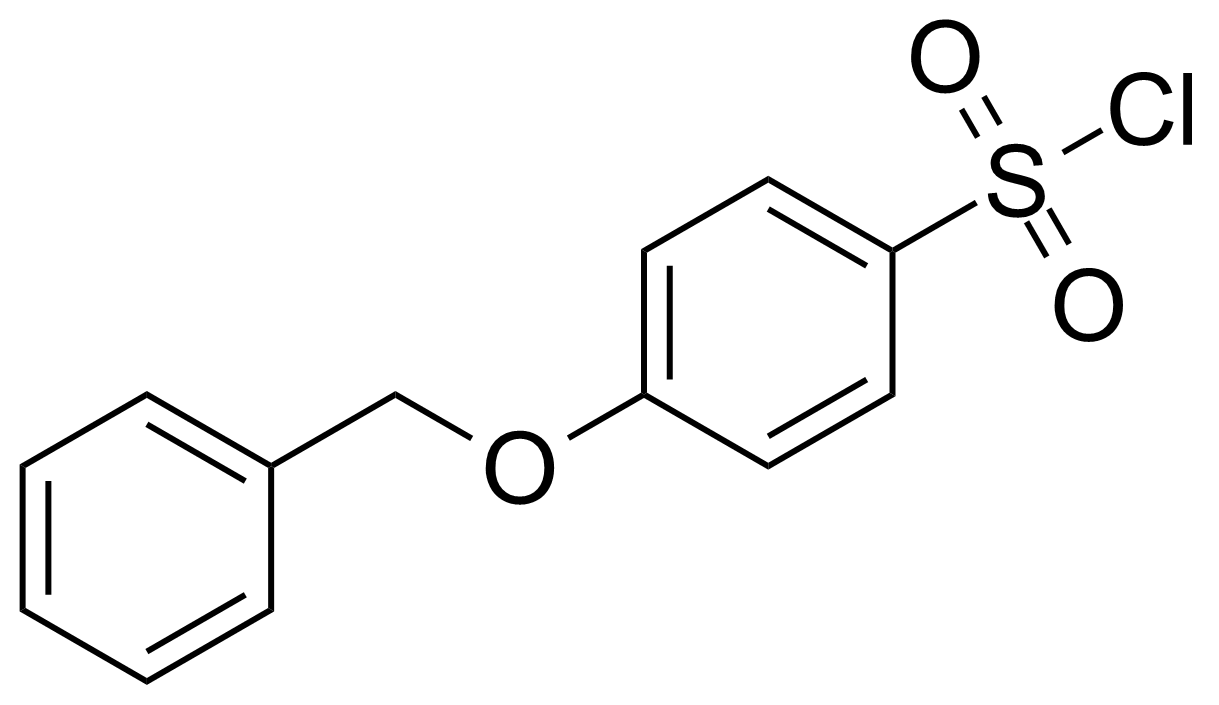 | [87001-32-9] | GEO-03974 | |
| 2-Bromobenzenesulfonyl chloride | 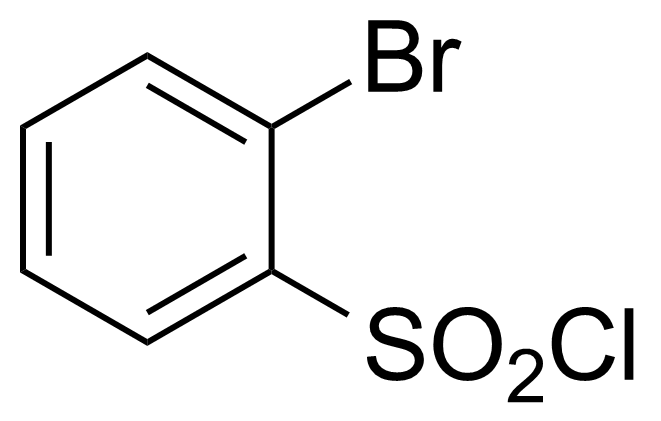 | [2905-25-1] | GEO-00385 | |
| (2-Bromo-4-chlorophenyl)methanesulfonyl chloride | 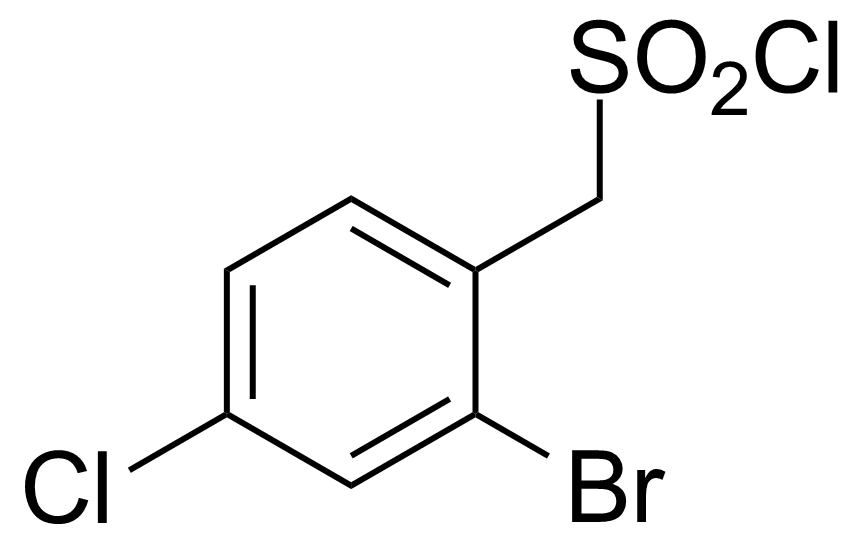 | [1427379-23-4] | GEO-03063 | |
| 4-Bromo-2,6-dichlorobenzenesulfonyl chloride | 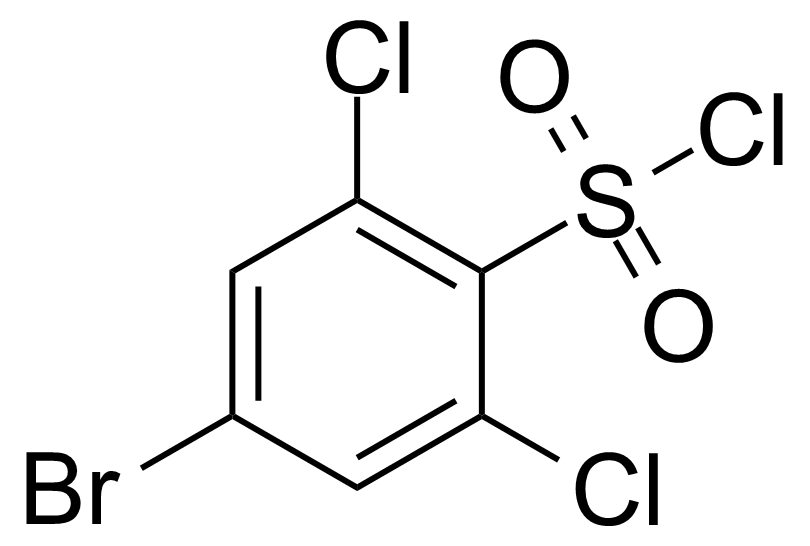 | [351003-54-8] | GEO-04158 | |
| 4-Bromo-2,6-difluorobenzenesulphonyl chloride | 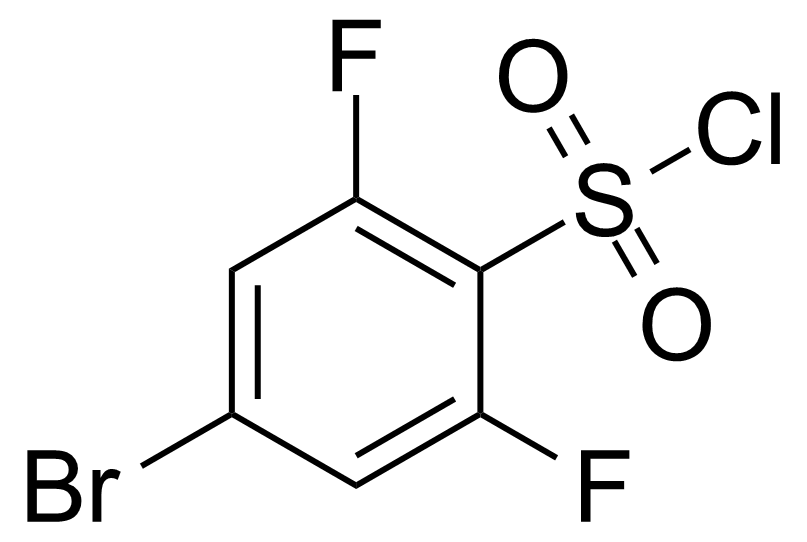 | [874804-21-4] | GEO-04016 | |
| 4-Bromo-2-fluorobenzylsulphonyl chloride | 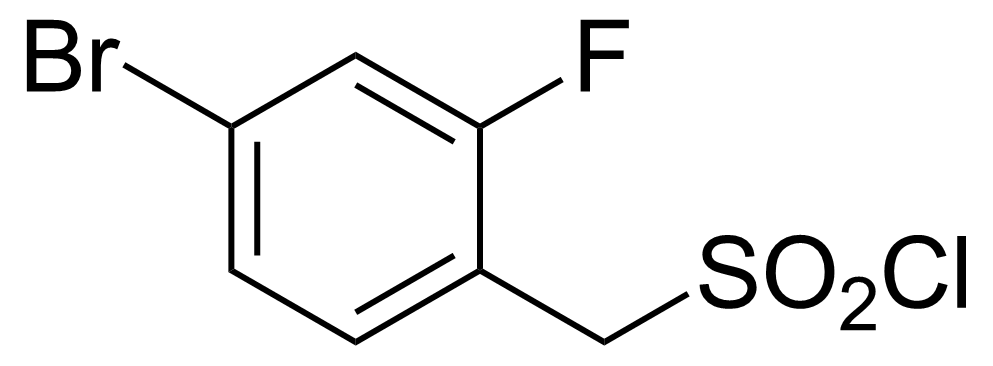 | [1178830-06-2] | GEO-03065 |