Oxiranes / Thiiranes
Oxiranes or epoxides are a class of compounds that contain a cyclic three atom ring ether. Thiiranes or episulfides are the sulfur analogues of an epoxides. They are less common and generally less stable than epoxides. Thiiranes can be prepared from epoxides by thiation using thiocyanate or thiourea. Most epoxides are generated by treating alkenes with peroxide-containing reagents (hydrogen peroxide, dimethyldioxirane, tert-butyl hydroperoxide) together with a metal complex catalyst. More typically for laboratory operations, the Prilezhaev reaction is employed, which involves the oxidation of the alkene with a peroxyacid such as m-CPBA. Chiral epoxides can be prepared enantioselectively from prochiral alkenes (Sharpless, Shi and Jacobsen asymmetric epoxidations). Epoxides can be also prepared from halohydrins by intramolecular SN2 substitution. Most reactions of oxiranes and thiiranes involve ring-opening with various nucleophiles. Epoxides are widely used in industry to assemble polymers known as epoxies or epoxy resins which are excellent adhesives and useful surface coatings.
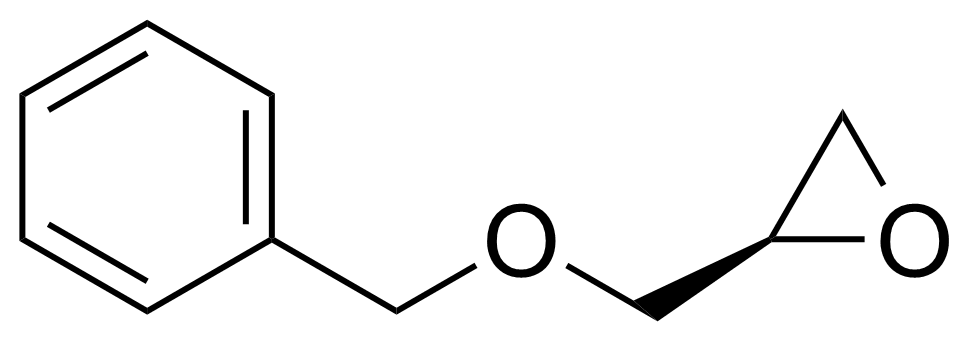
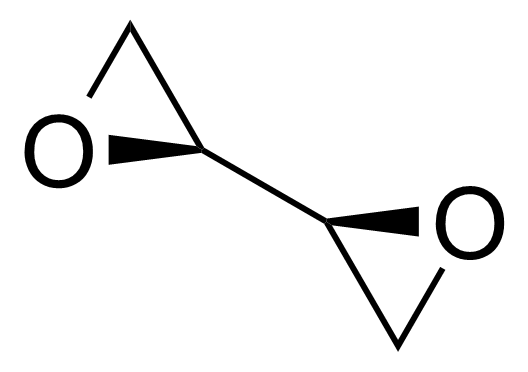

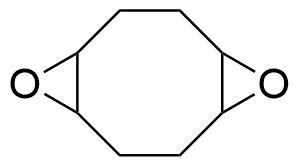

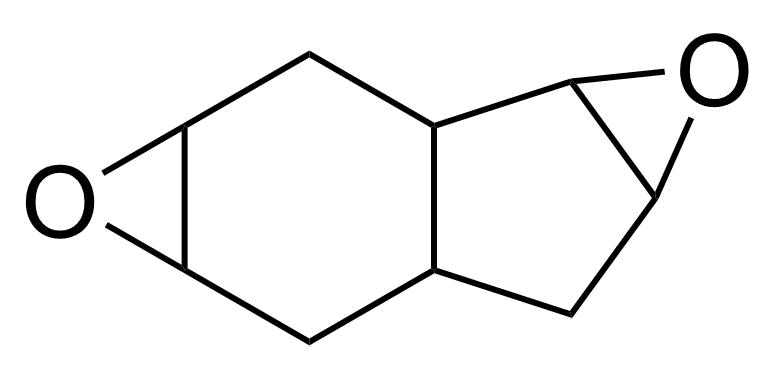
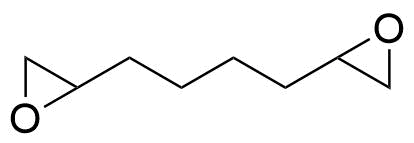



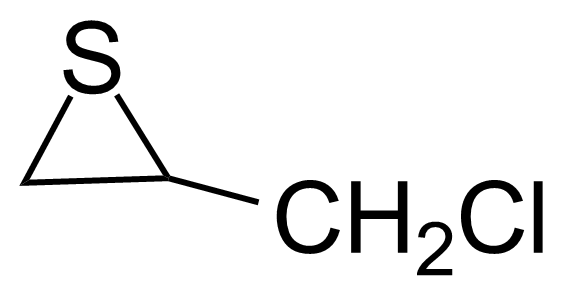


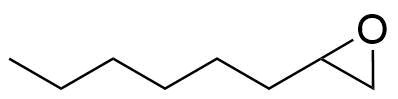


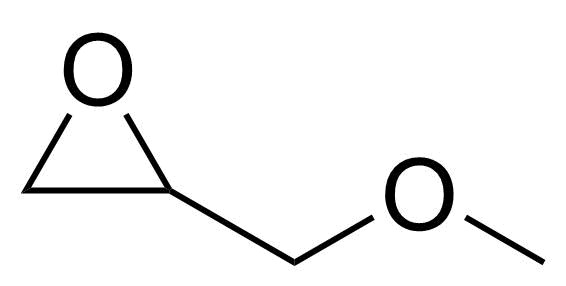
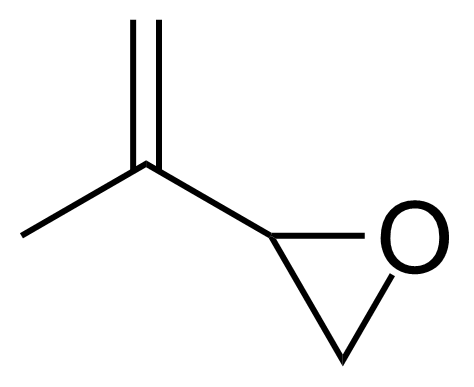
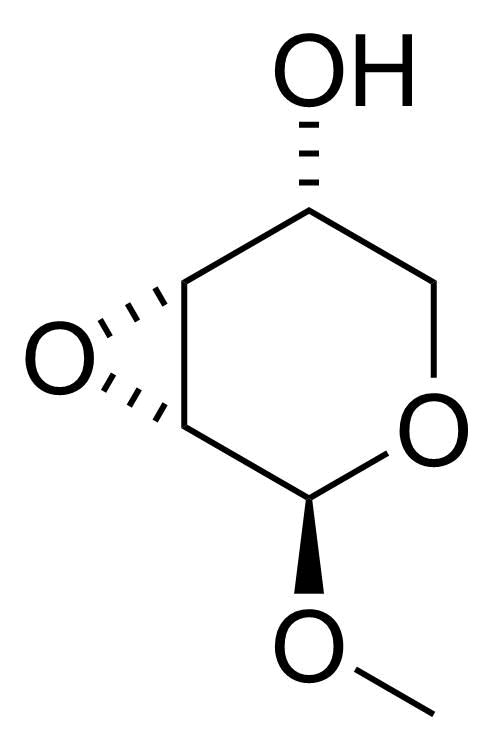
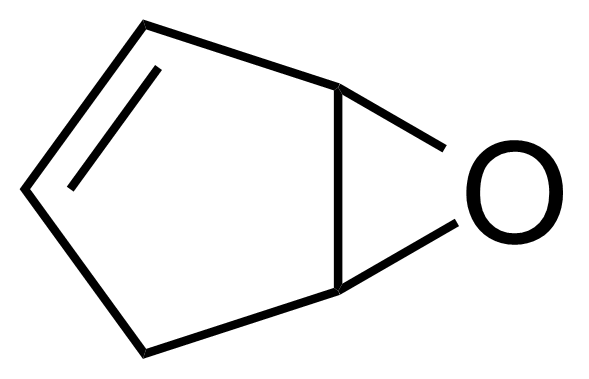
![Structure of (1S)-1-[(2R)-Oxiran-2-yl]prop-2-en-1-ol](https://georganics.sk/wp-content/uploads/2021/05/GEO-02737_1S-1-2R-Oxiran-2-ylprop-2-en-1-ol.png)
![Structure of (1R)-1-[(2S)-Oxiran-2-yl]prop-2-en-1-ol](https://georganics.sk/wp-content/uploads/2021/05/GEO-02738_1R-1-2S-Oxiran-2-ylprop-2-en-1-ol.png)