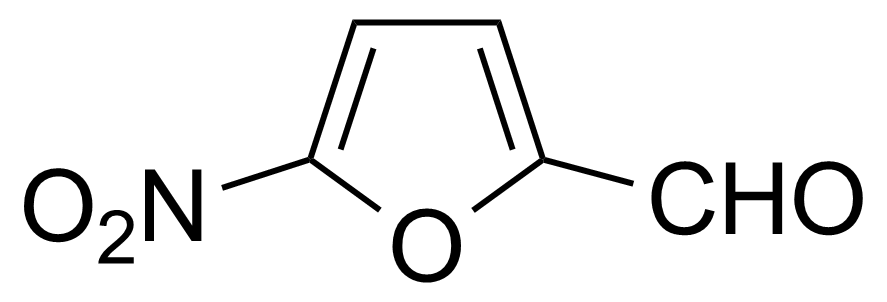
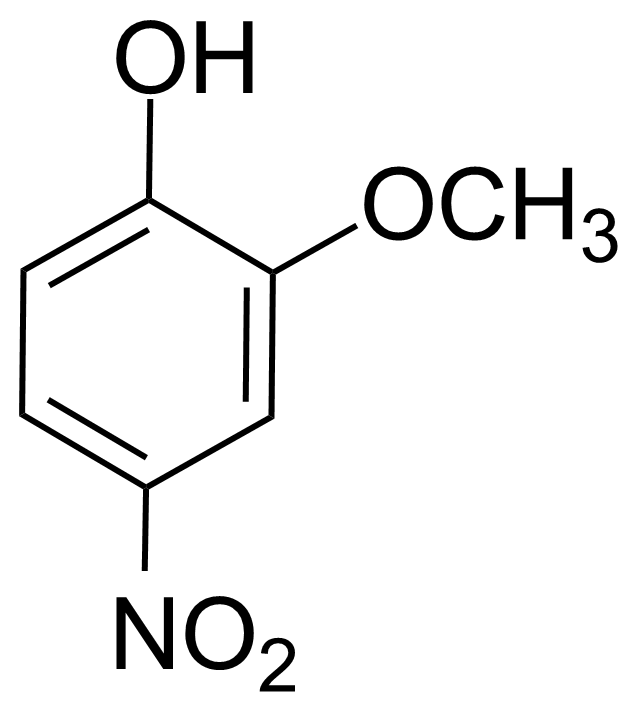
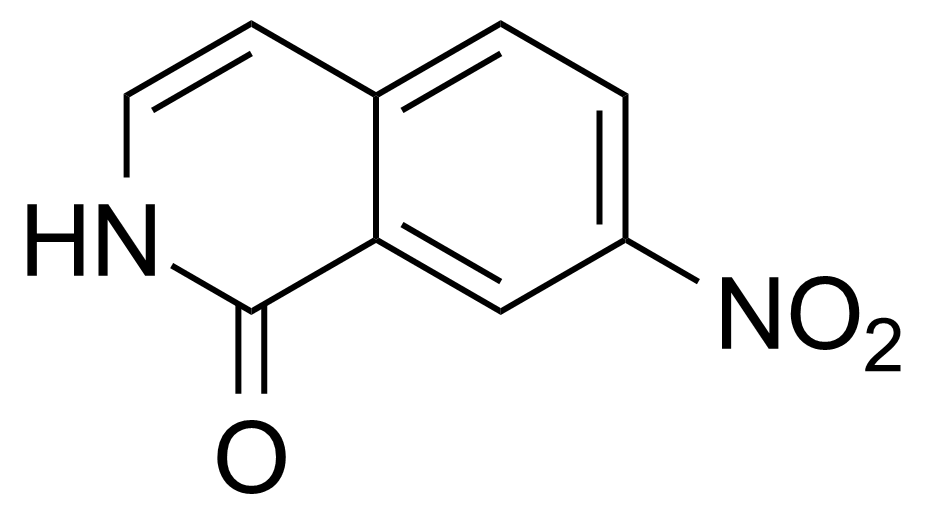
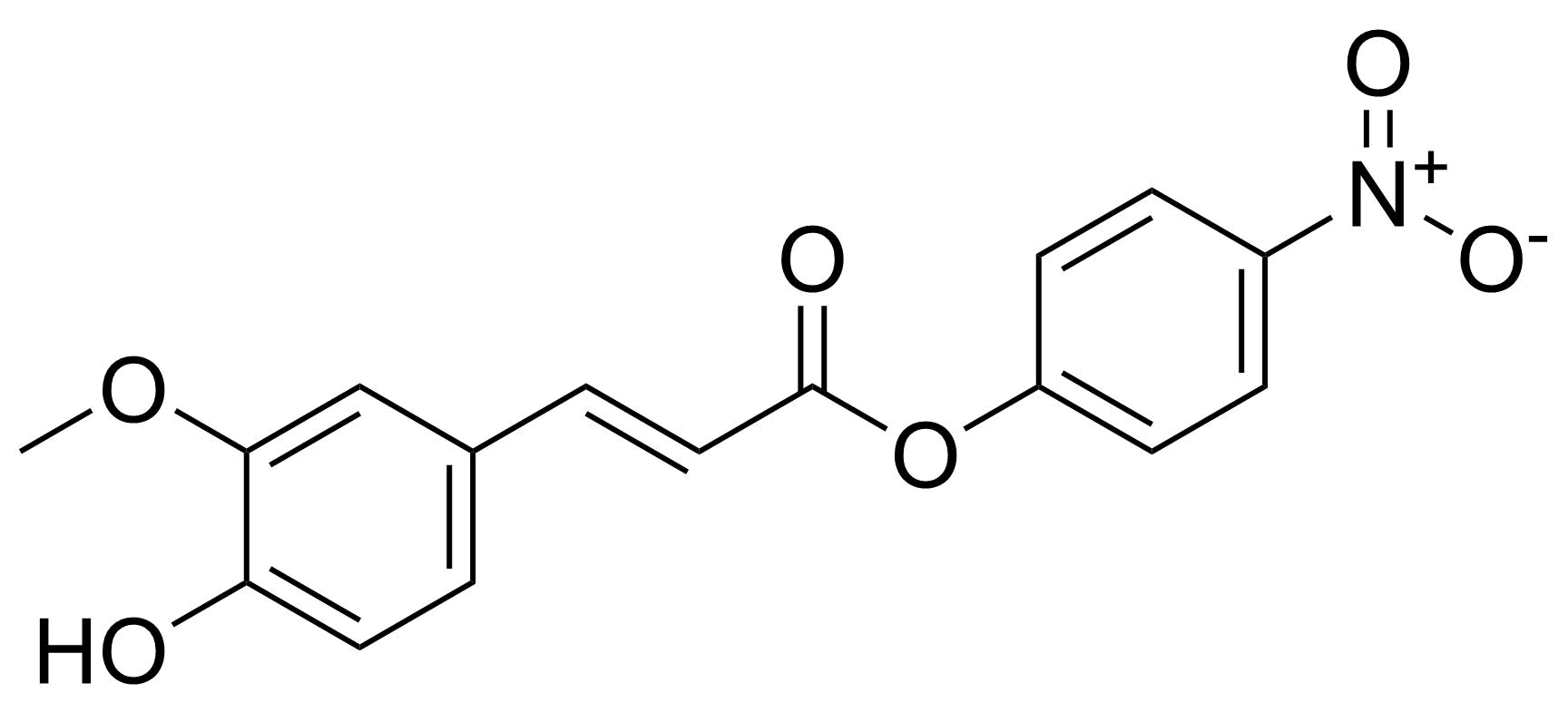
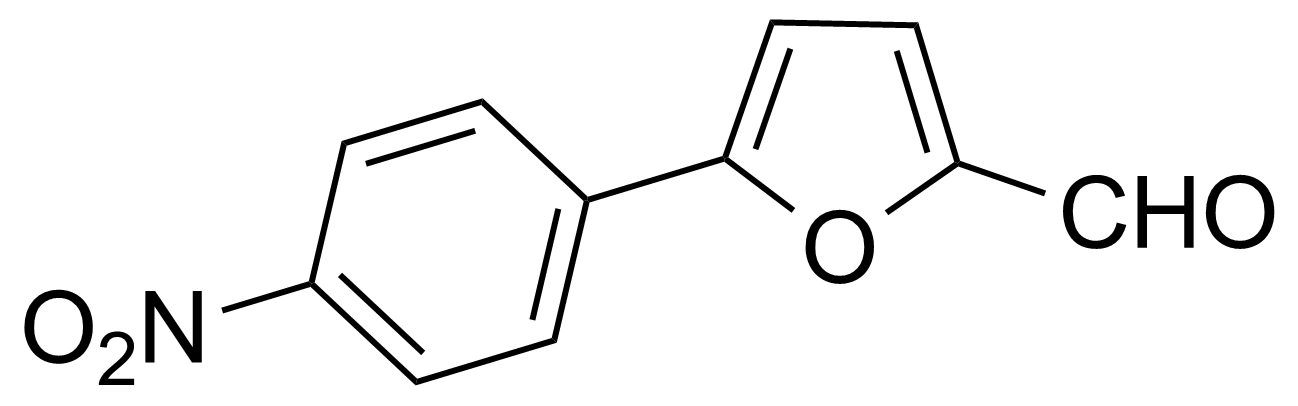
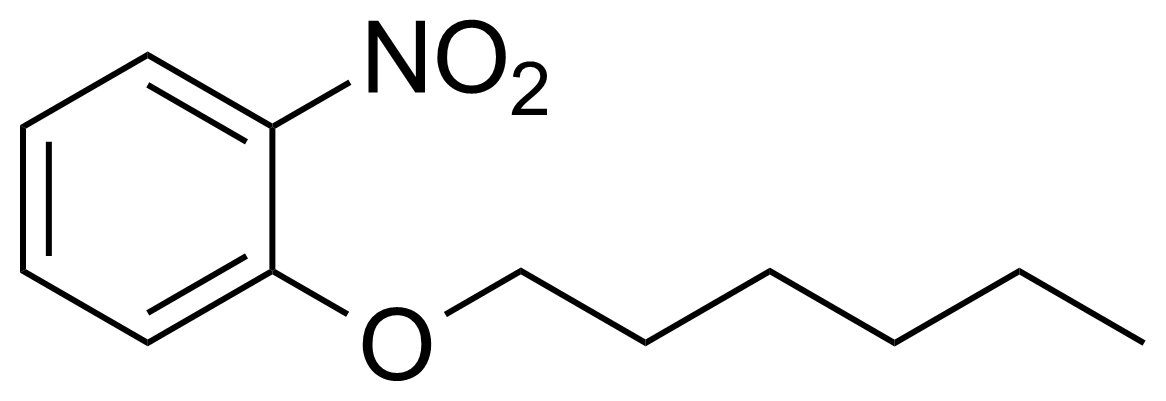
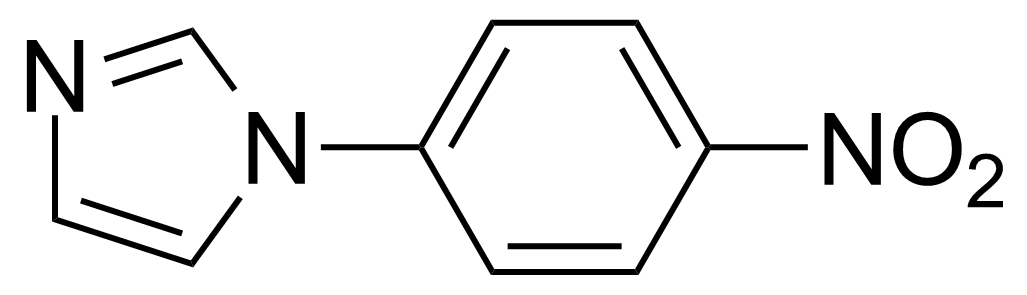
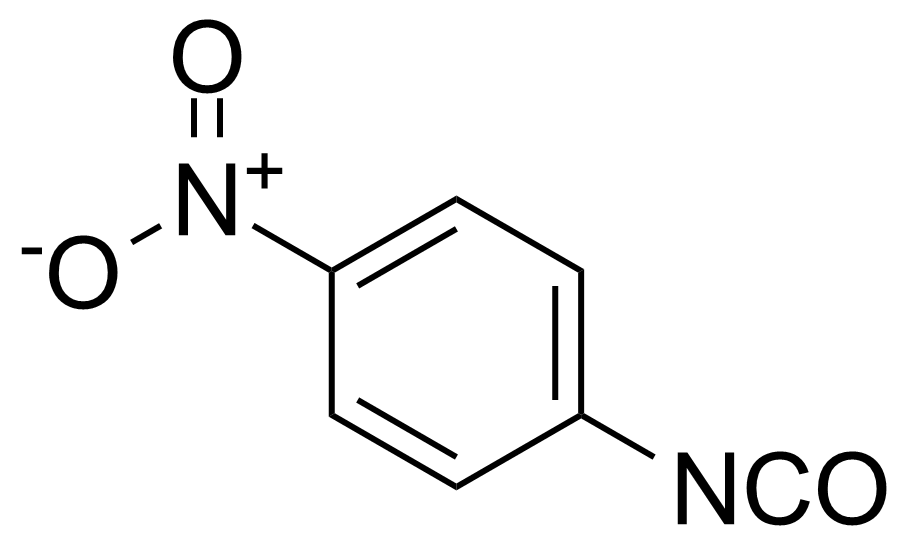
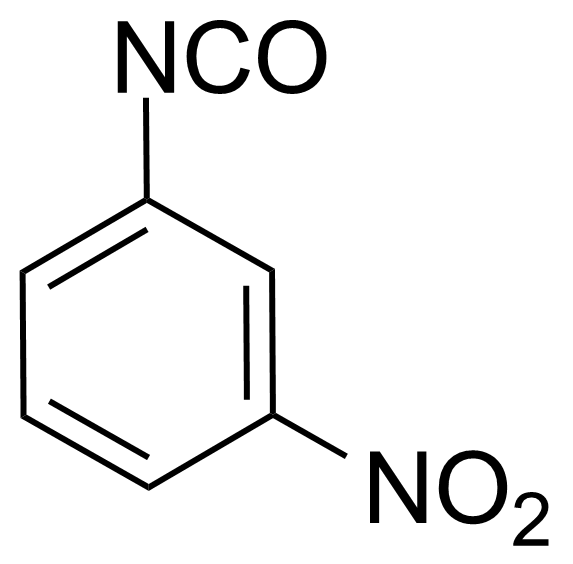
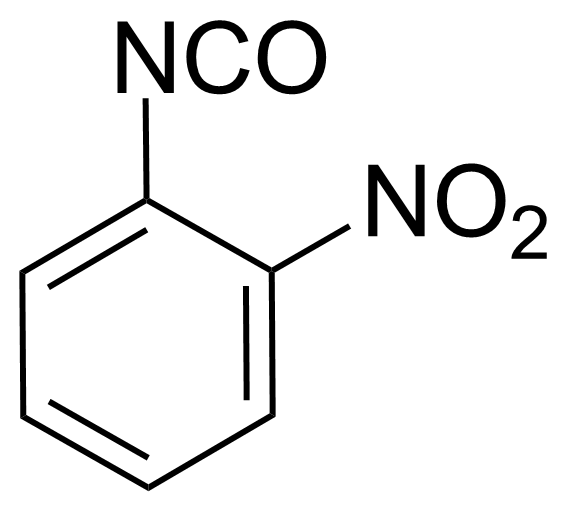
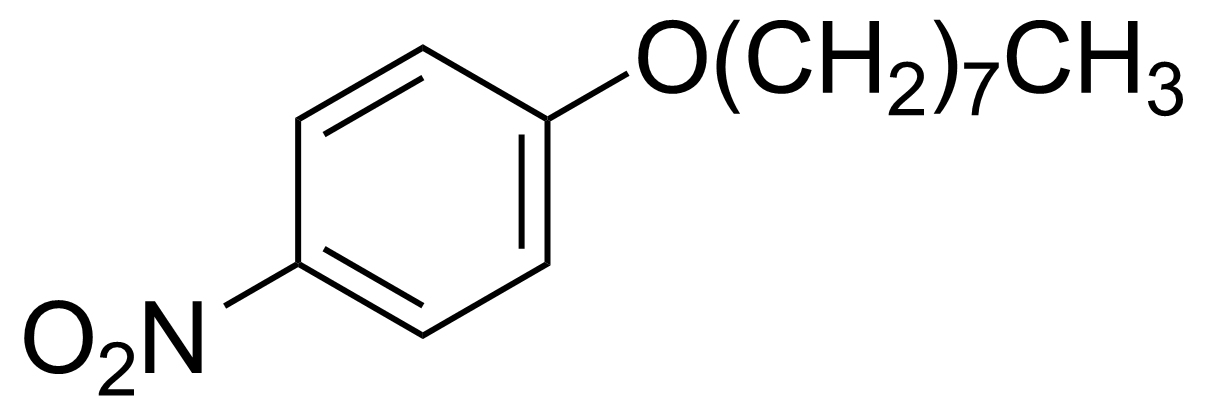
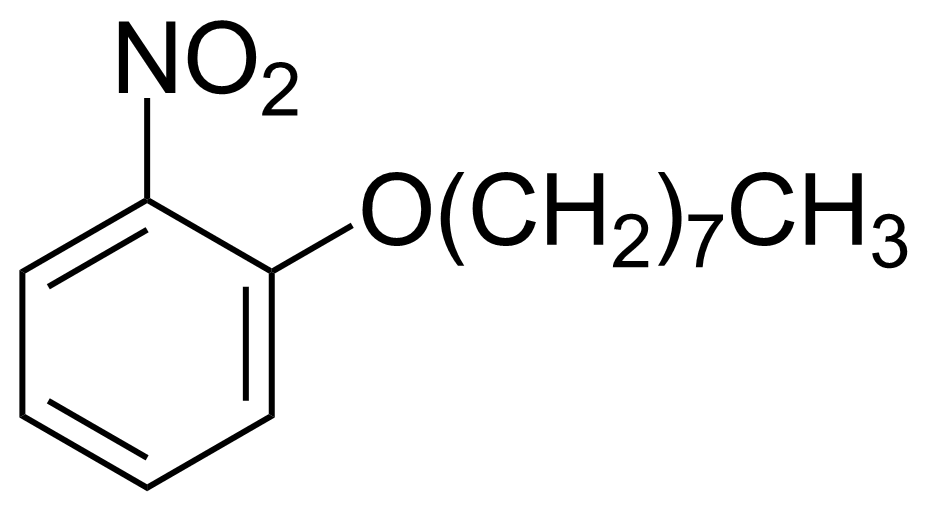Nitro group-containing compounds
Nitro group-containing compounds are organic compounds that contain at least one nitro functional group. The nitro group is one of the most common explosophores and it is also strongly electron-withdrawing. The presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature, chloramphenicol is a rare example. Aromatic nitro compounds are typically synthesized by nitration using a mixture of nitric acid and sulfuric acid. Aliphatic nitro compounds can be synthesized by various methods (radical nitration of alkanes, nucleophilic substitution between halocarbons and metal nitrite salts, oxidation of oximes or primary amines, decarboxylation of α-nitro carboxylic acids etc.). Nitro compounds participate in several organic reactions, the most important being their reduction to the corresponding amines. Protons in alpha position to the nitro group are acidic and therefore they can react in additions like nitroaldol (Henry) or Michael reaction.
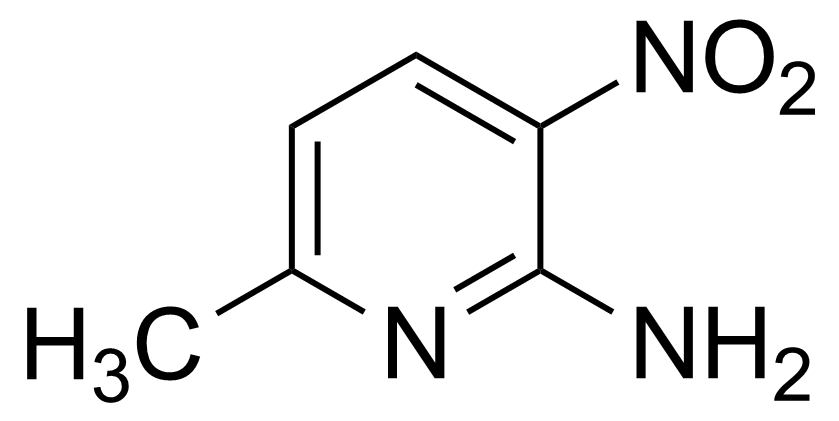
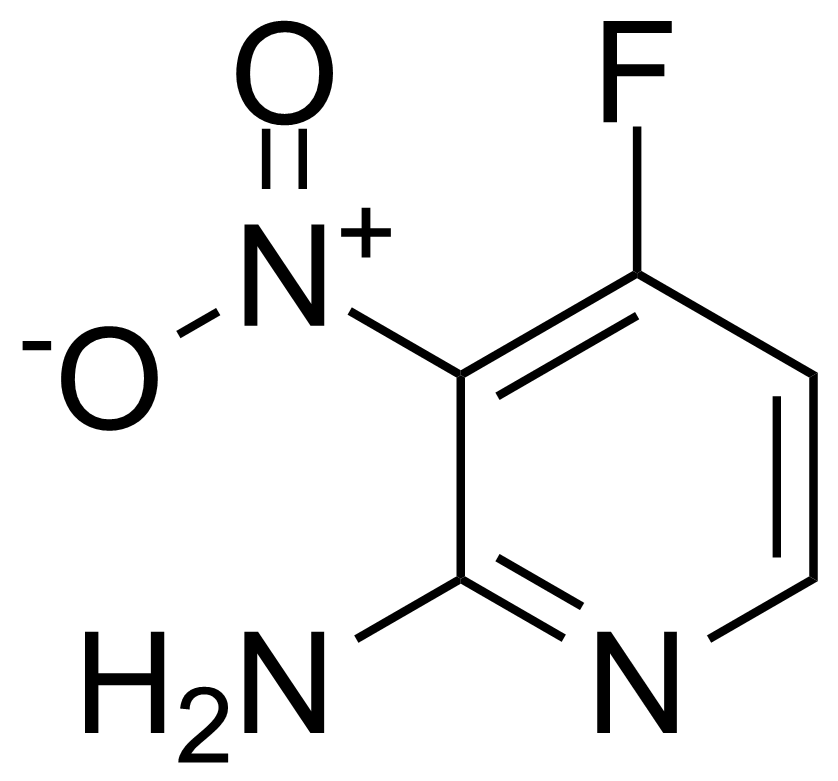
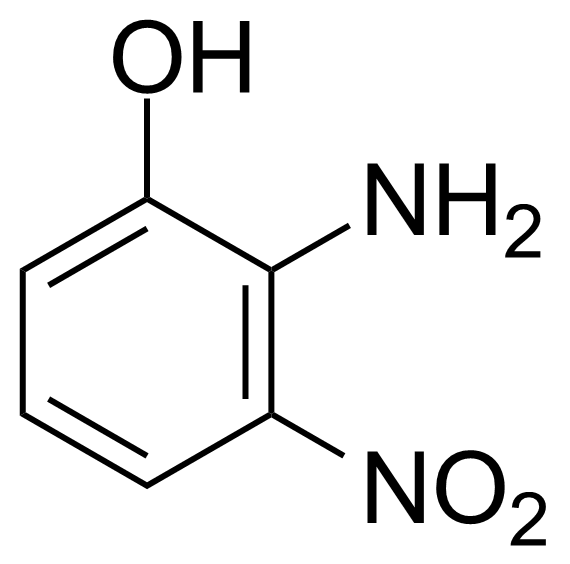
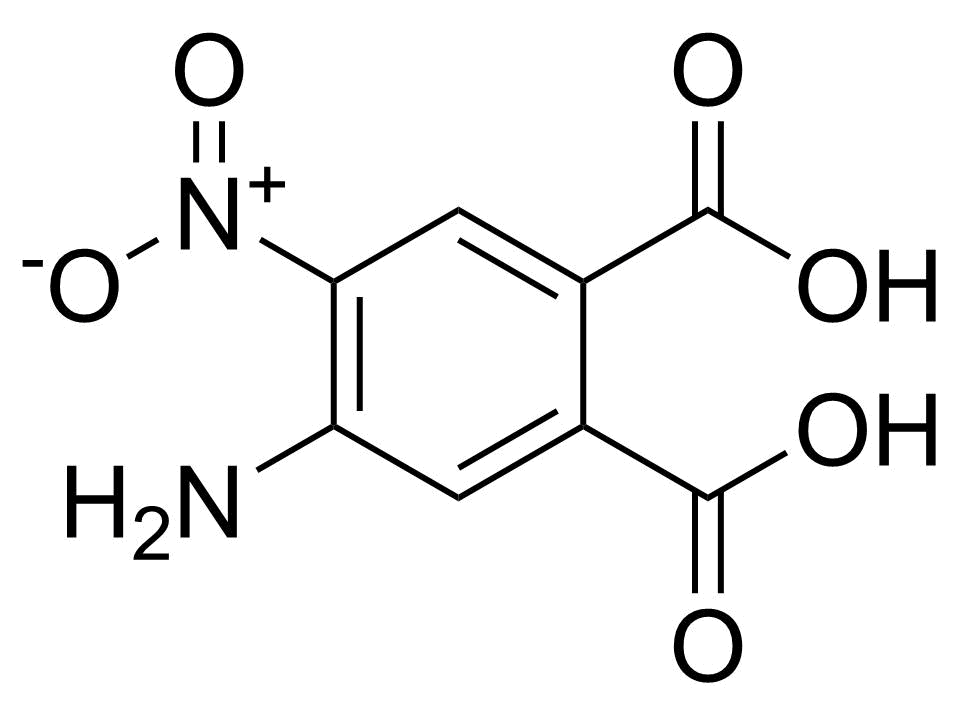
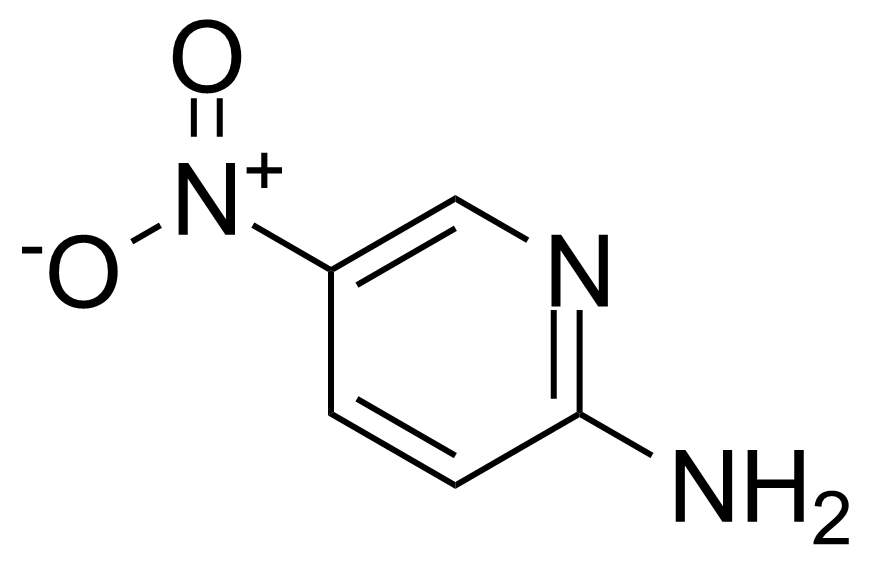
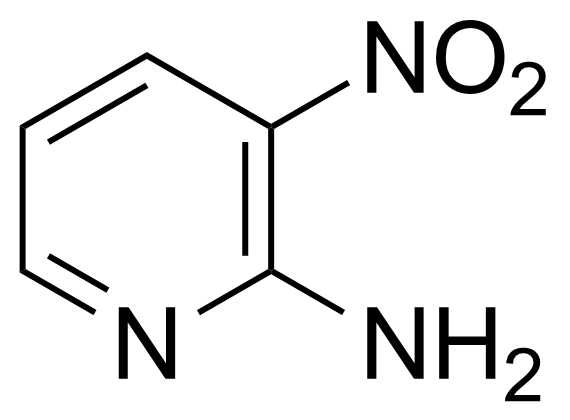
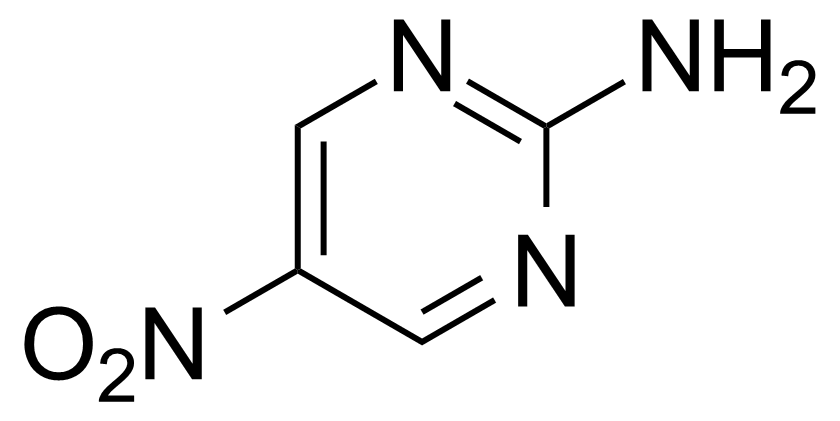
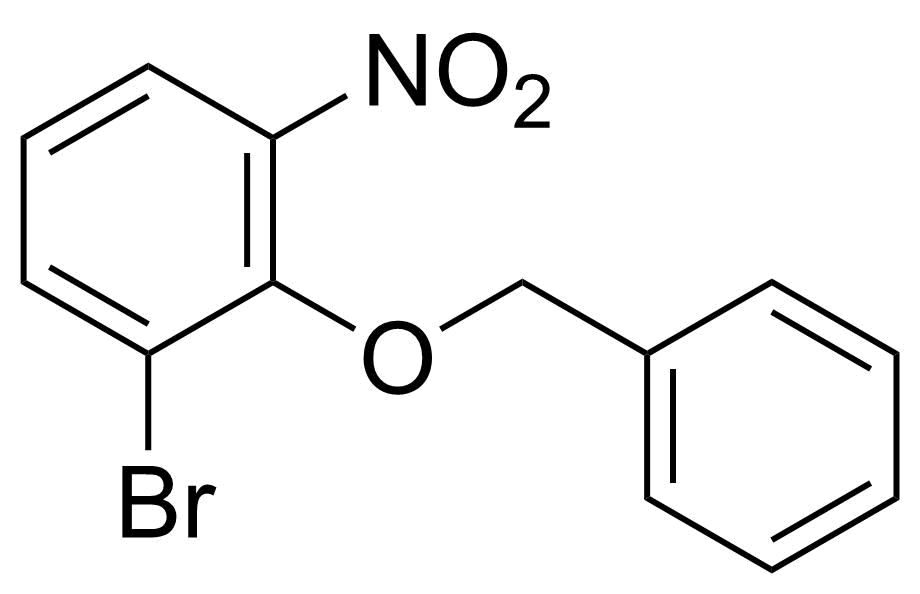
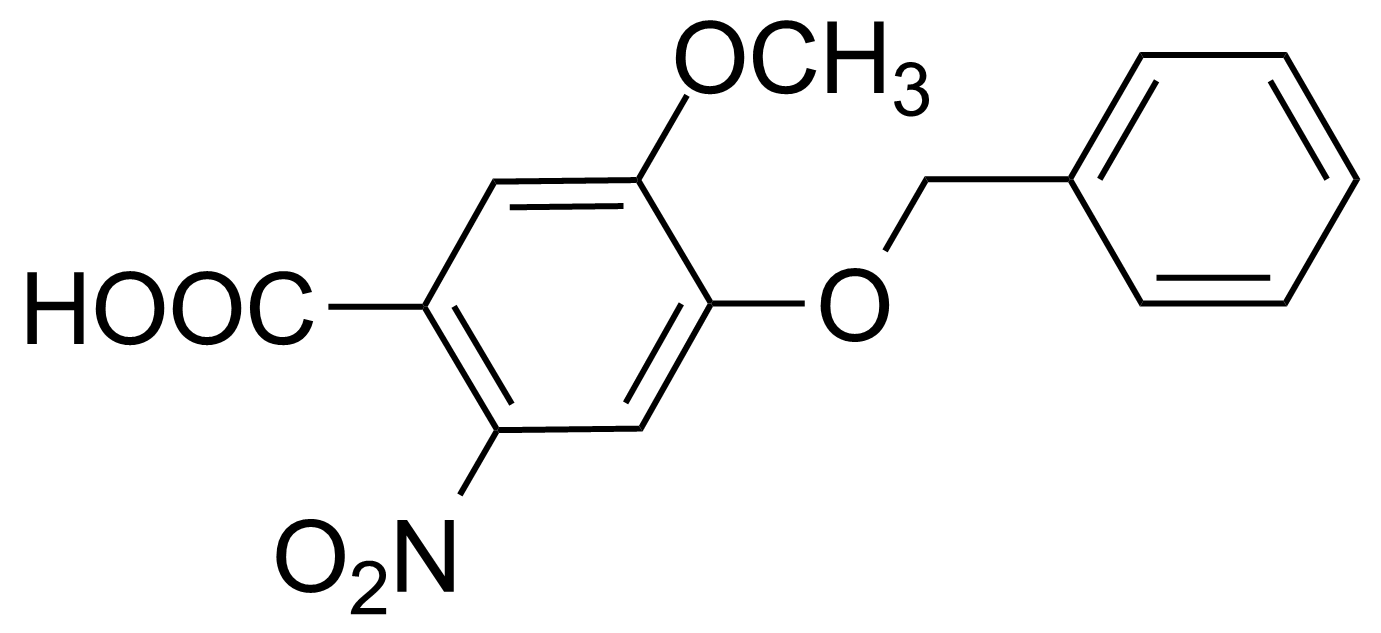
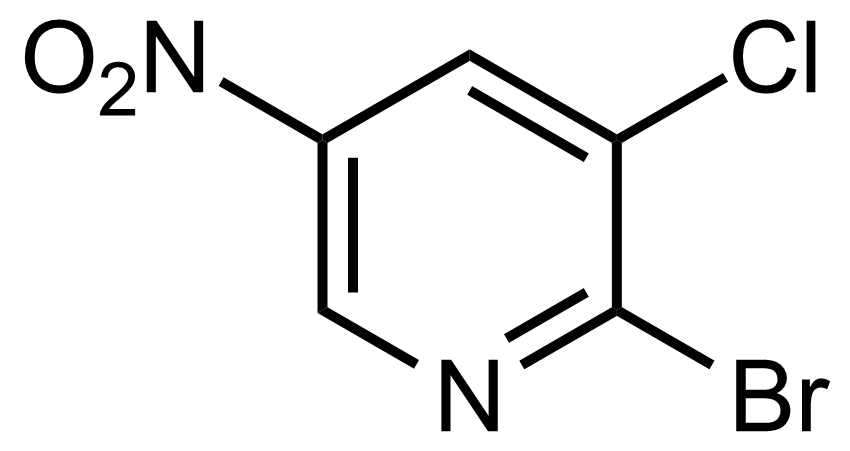
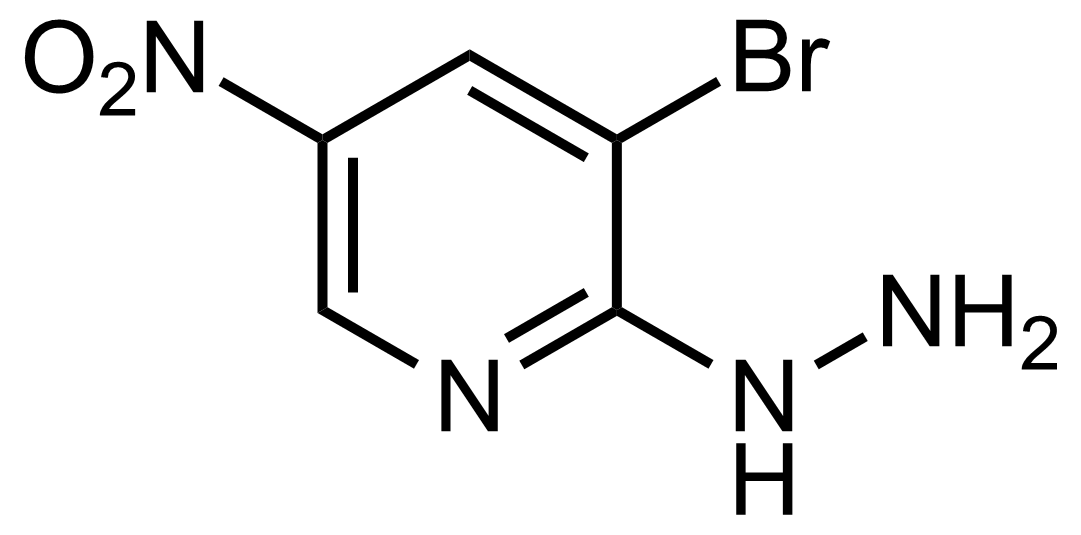
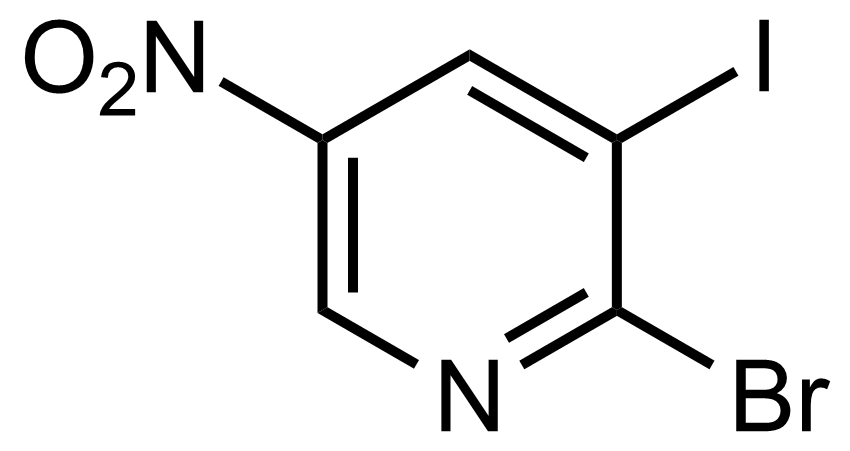
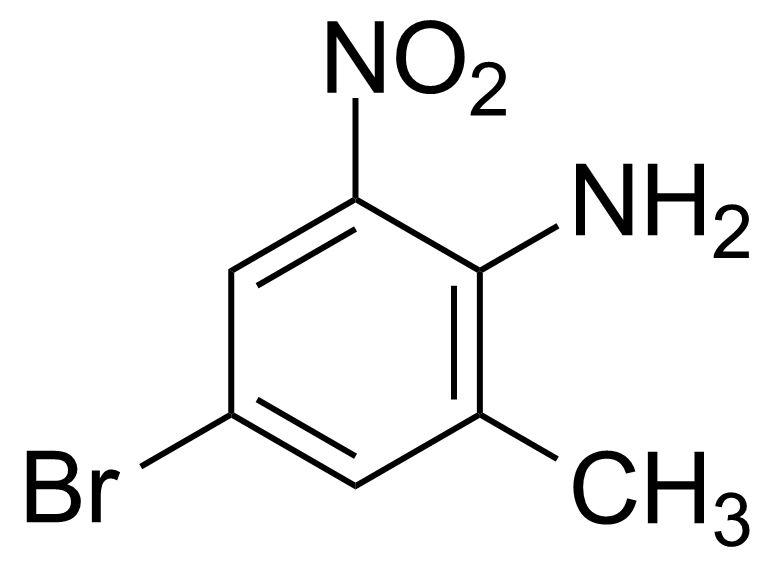
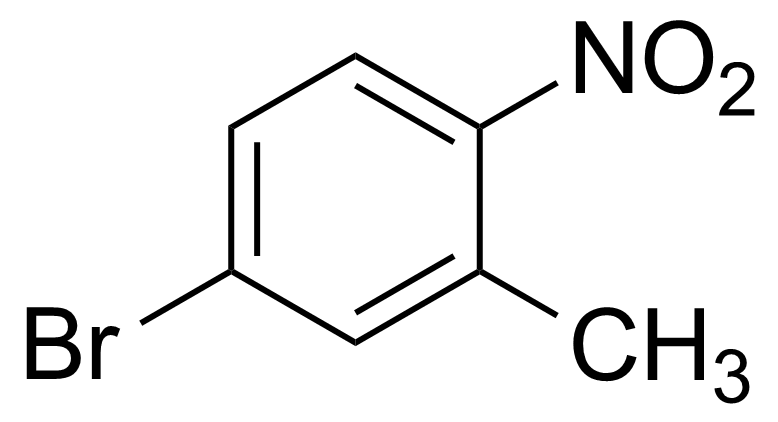
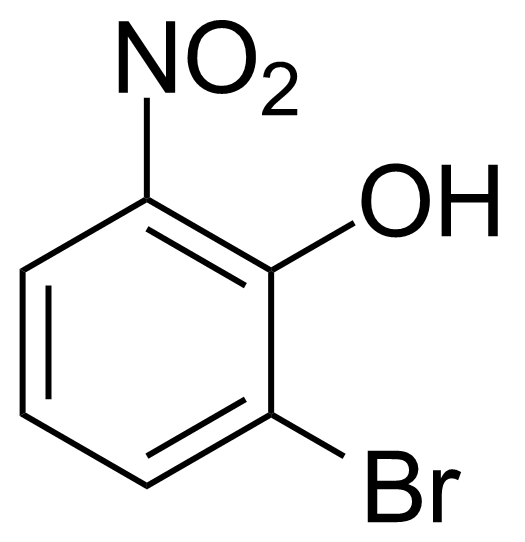
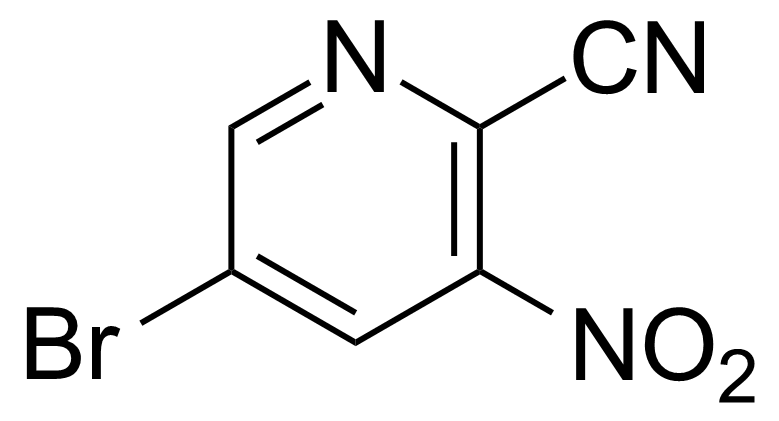
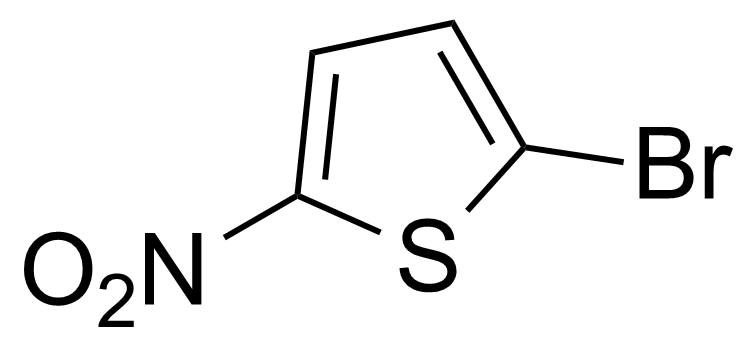
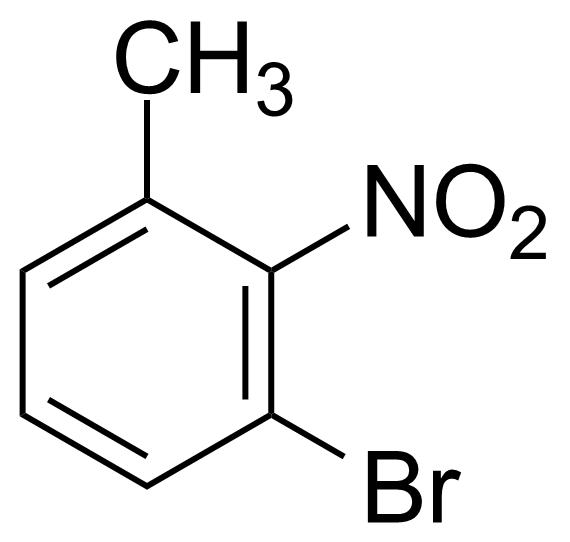
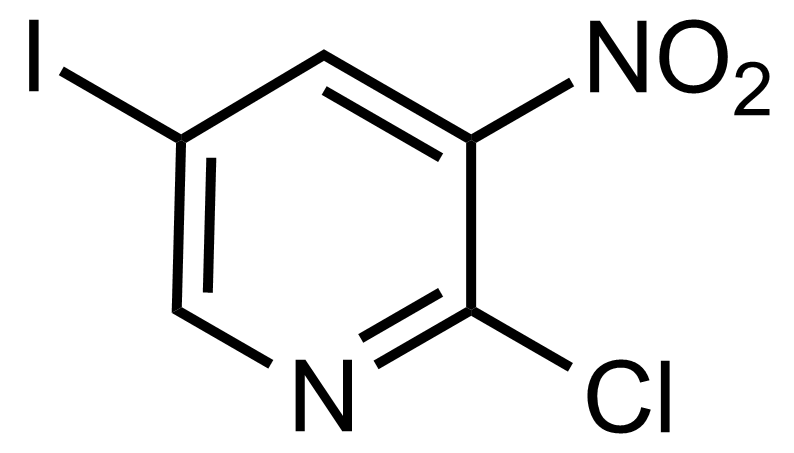
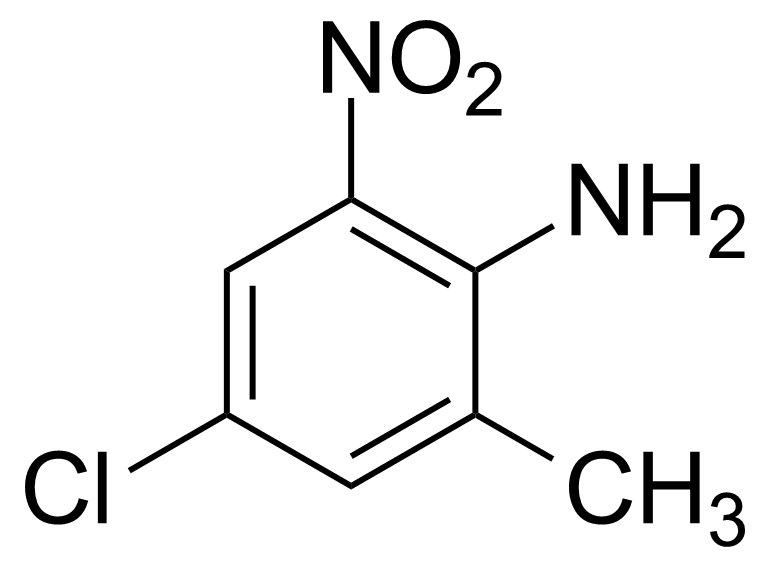
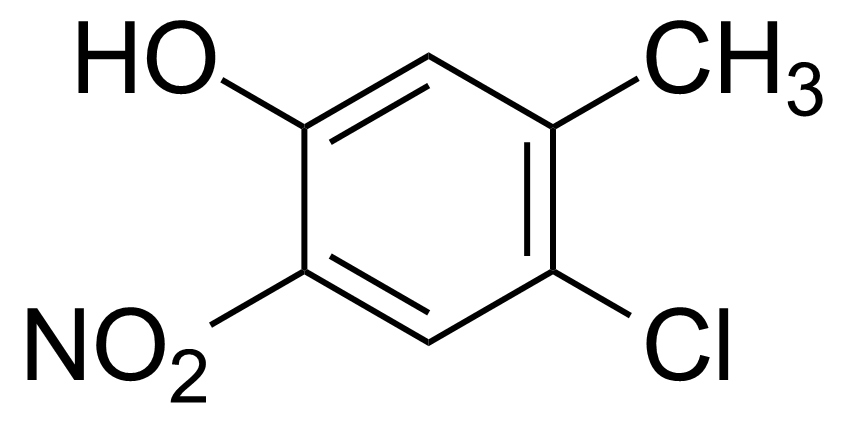
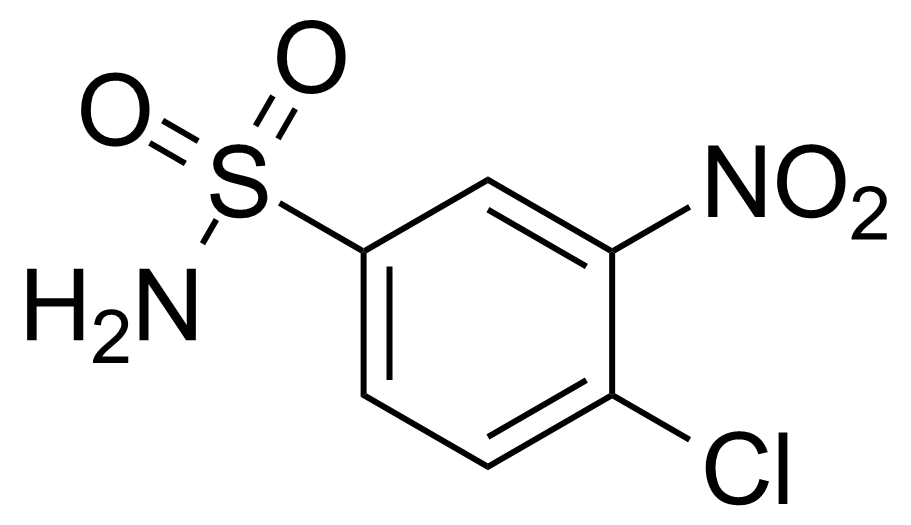
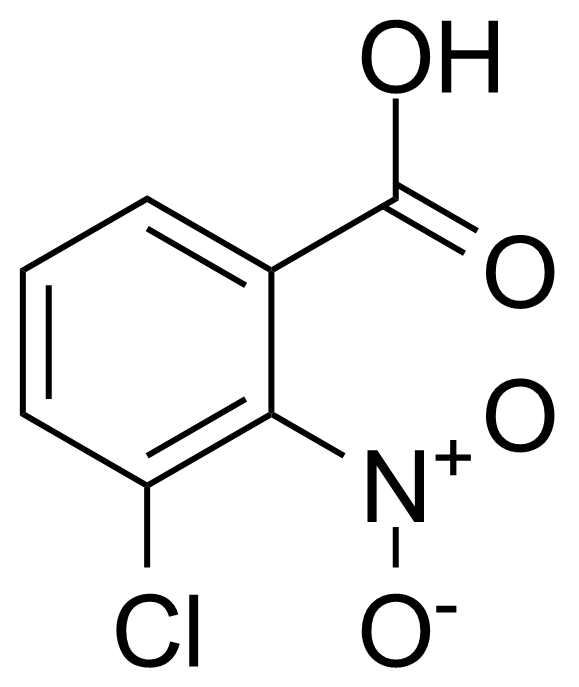


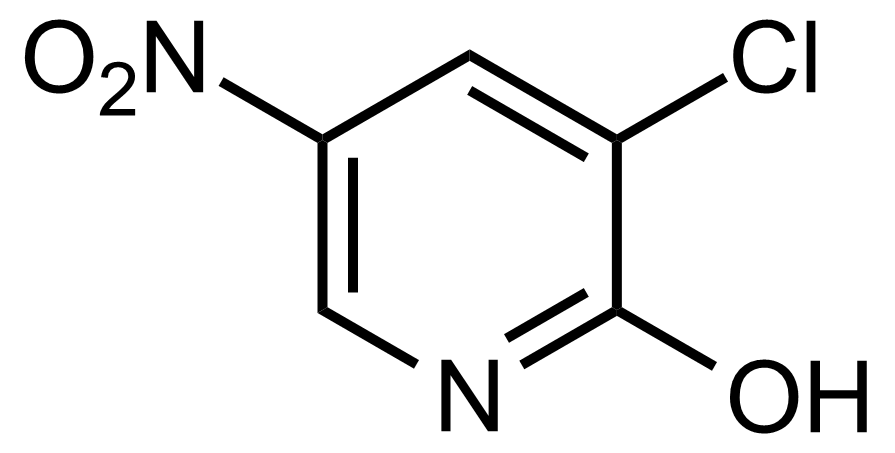


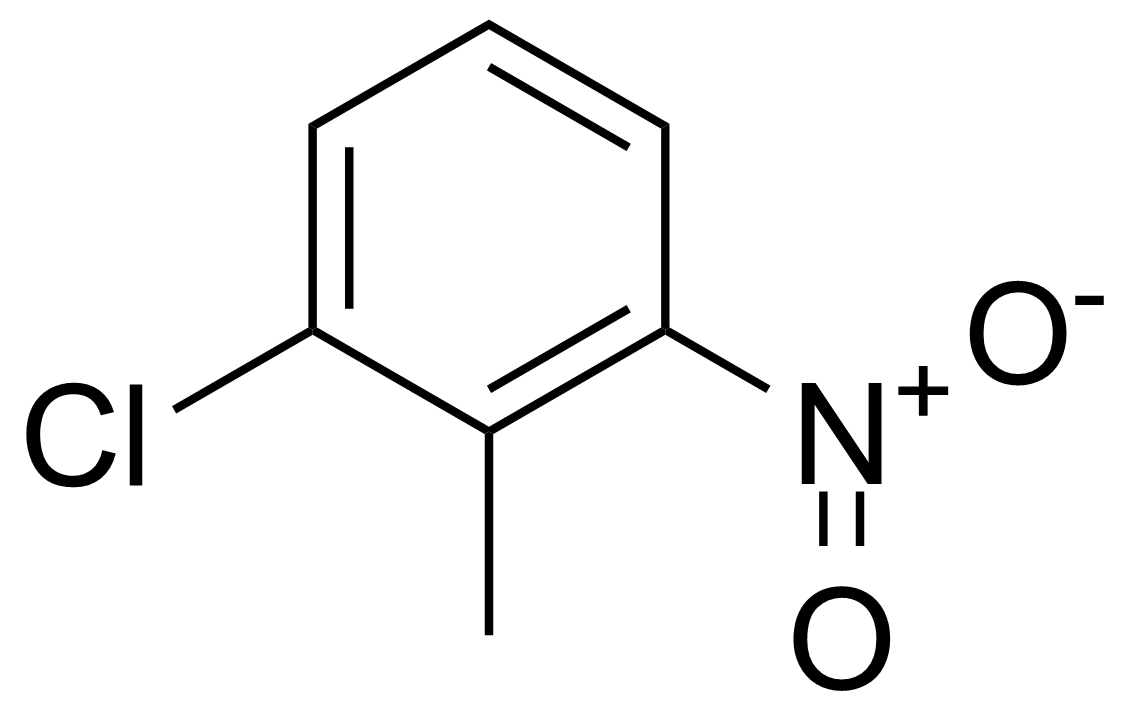

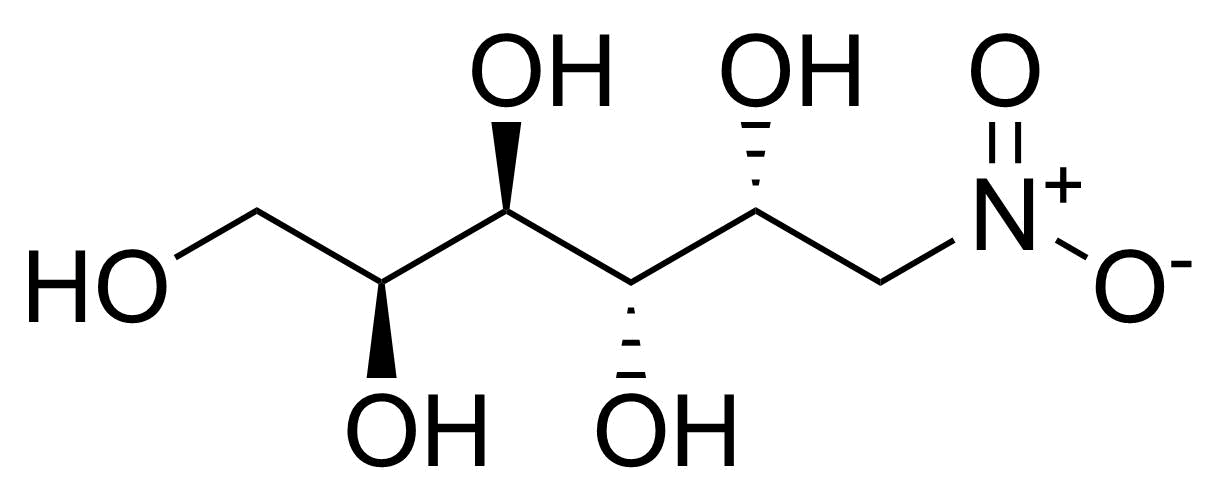
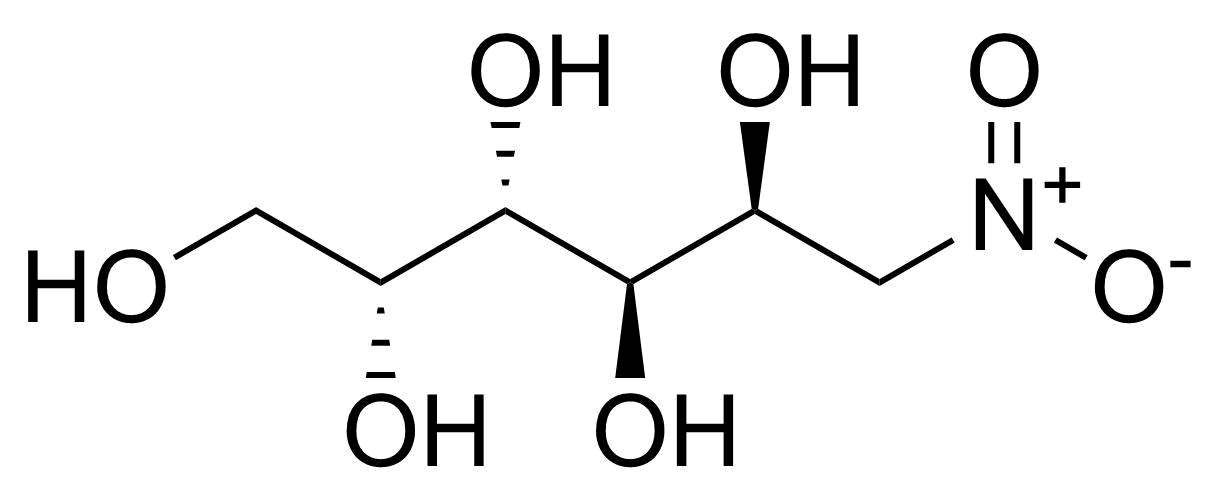
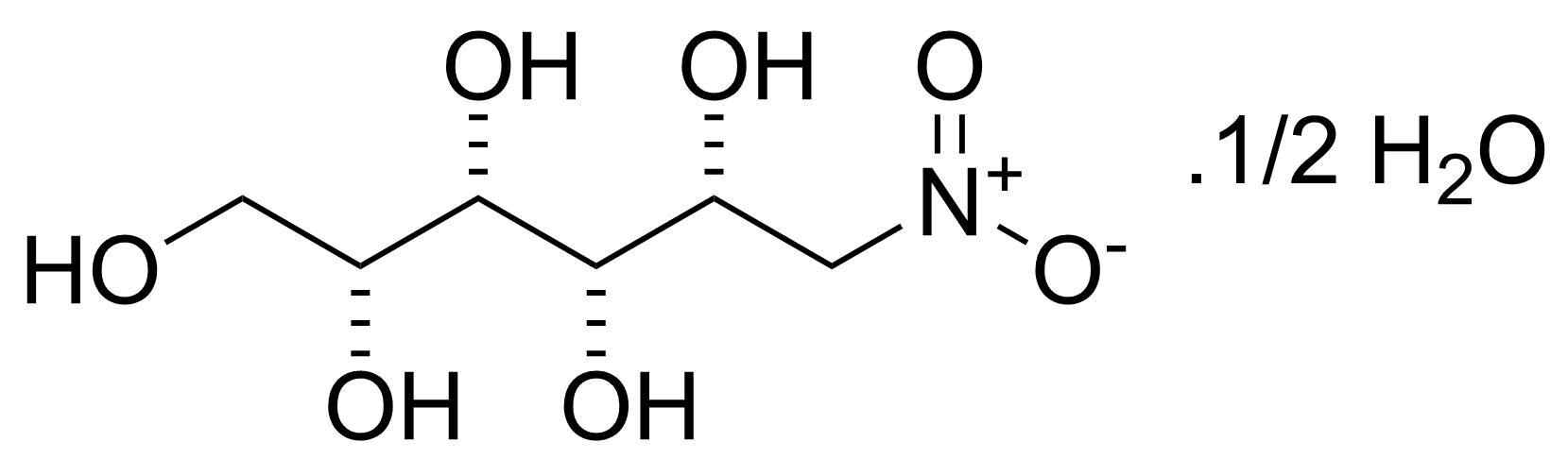
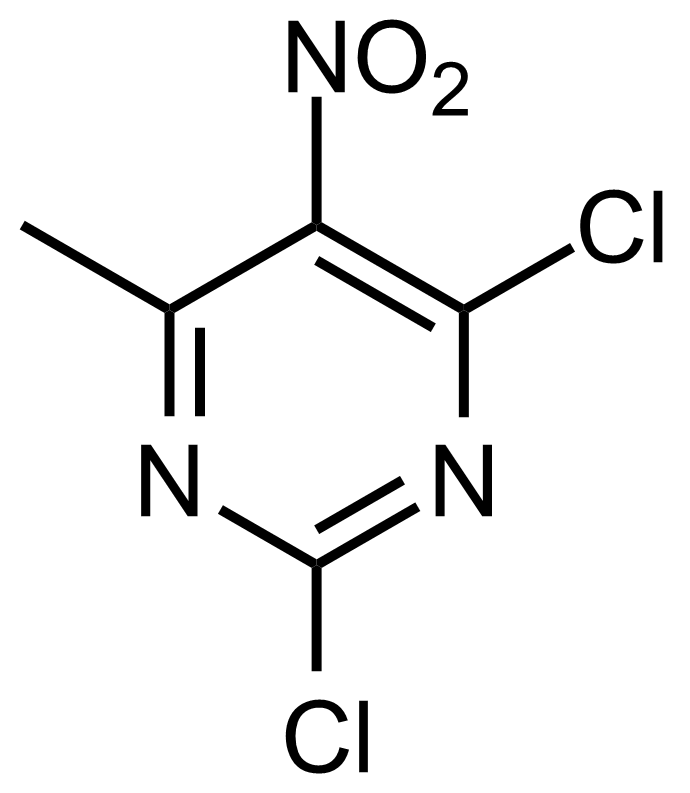
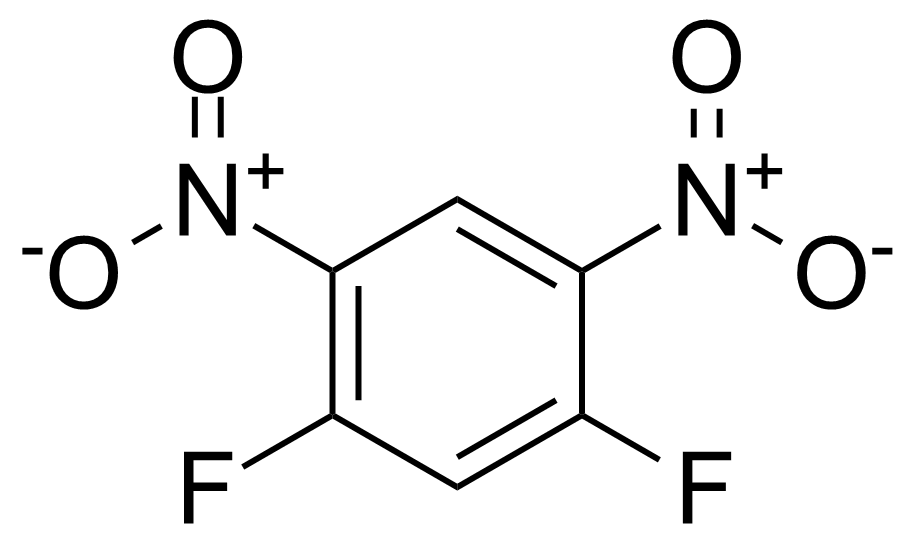
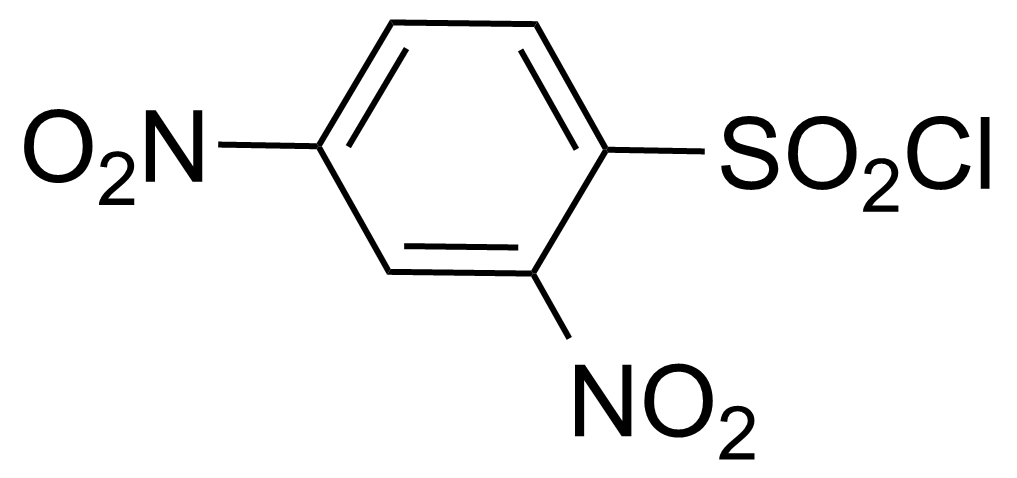
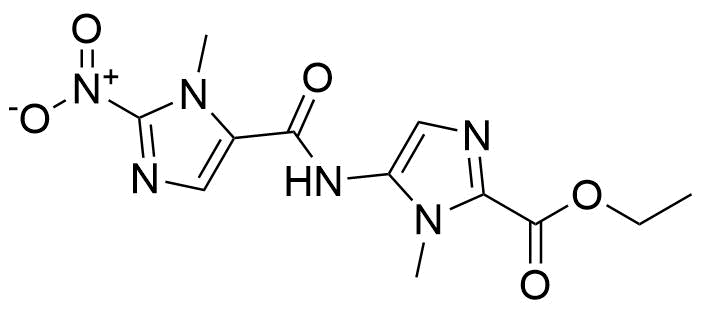
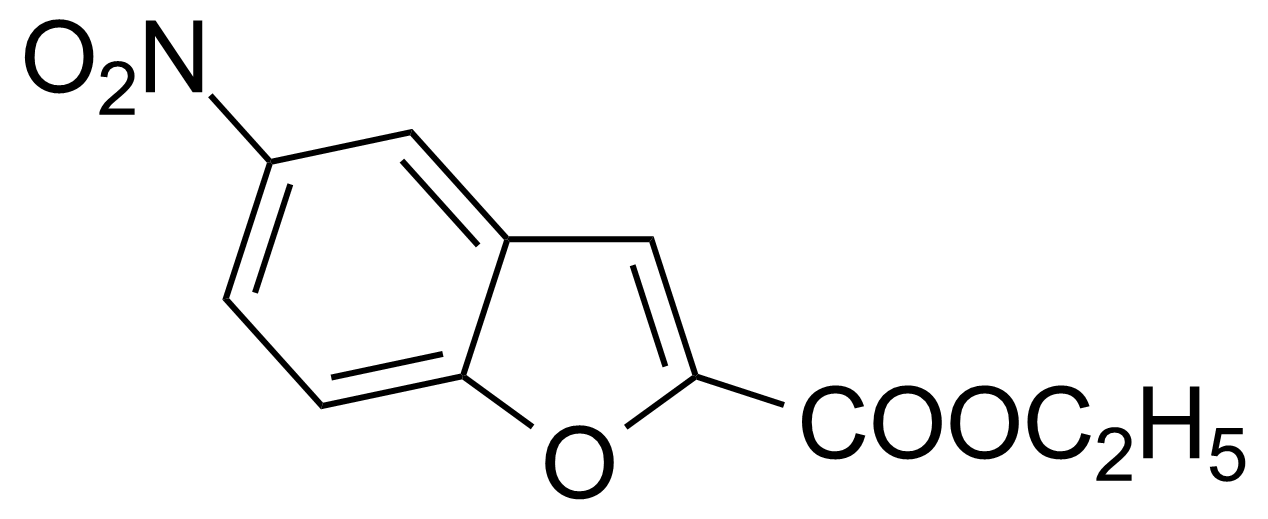
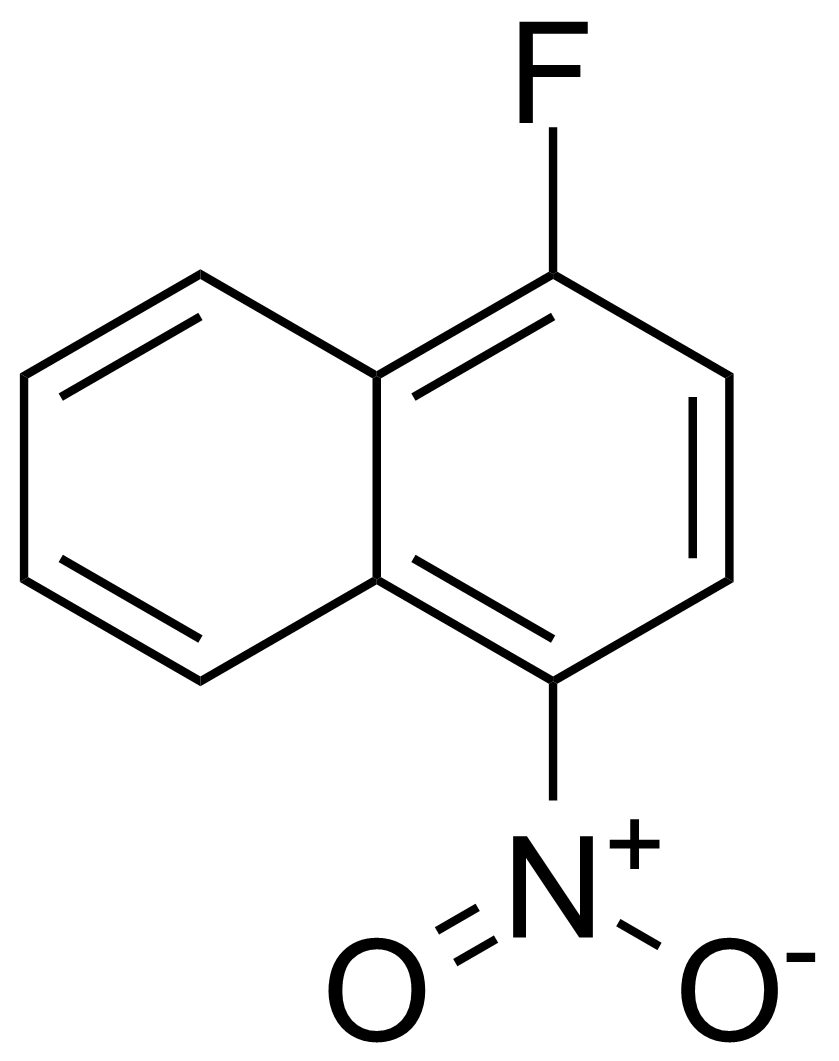
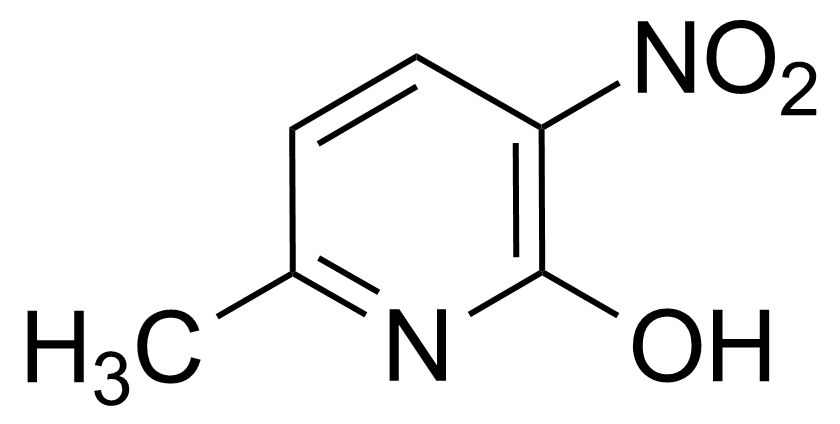
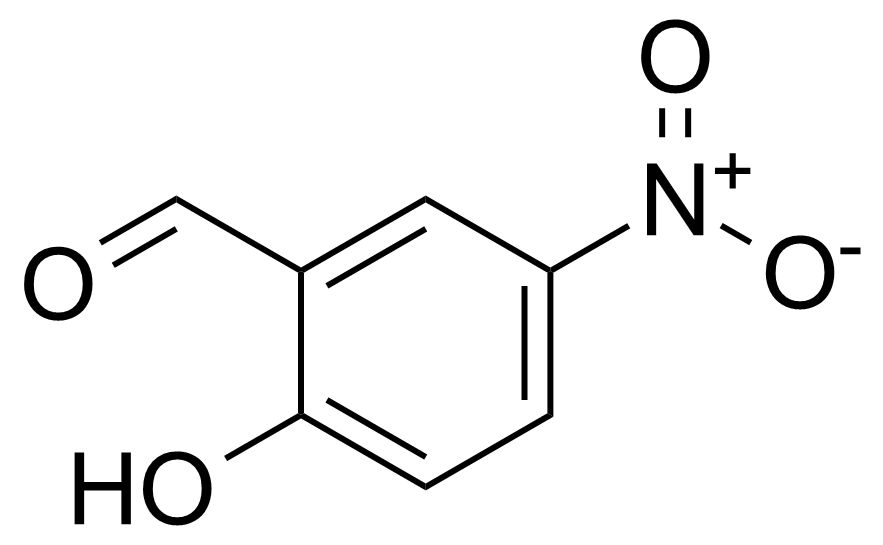
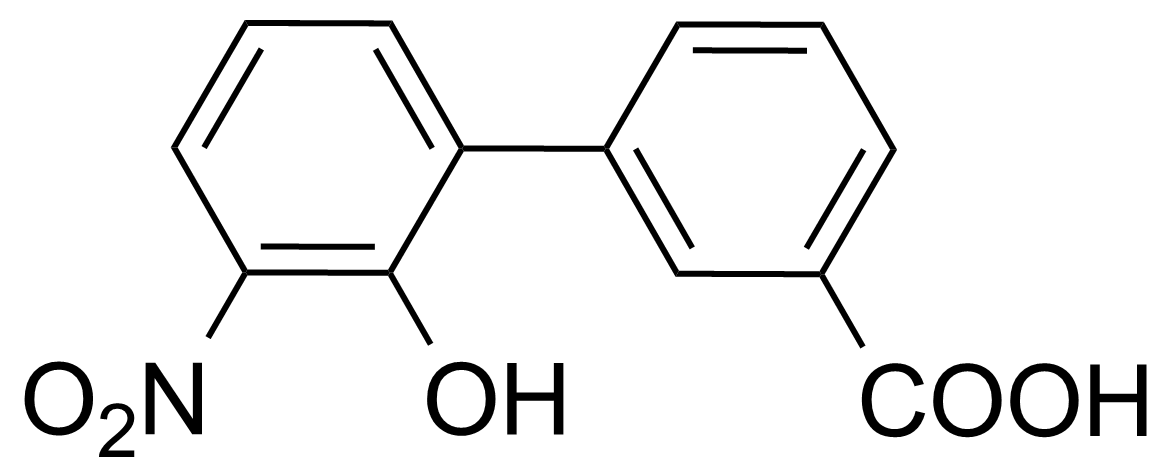
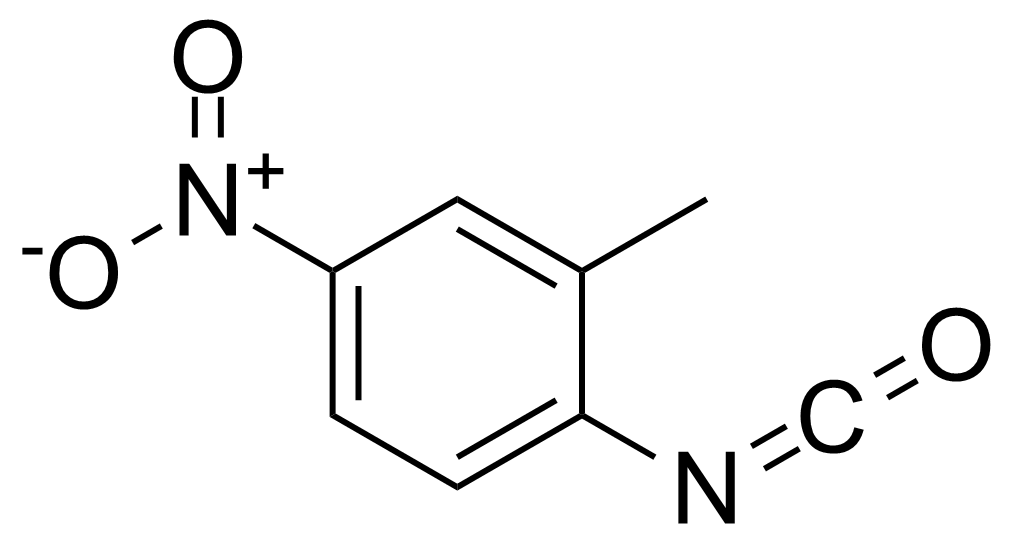
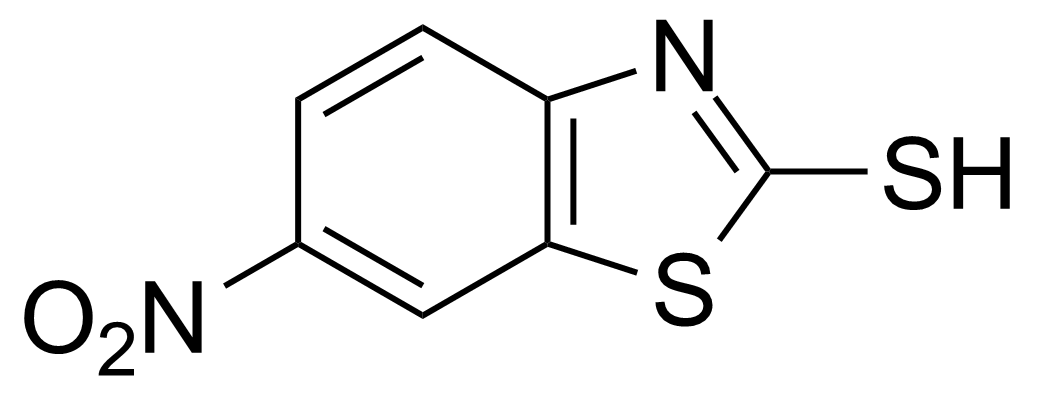
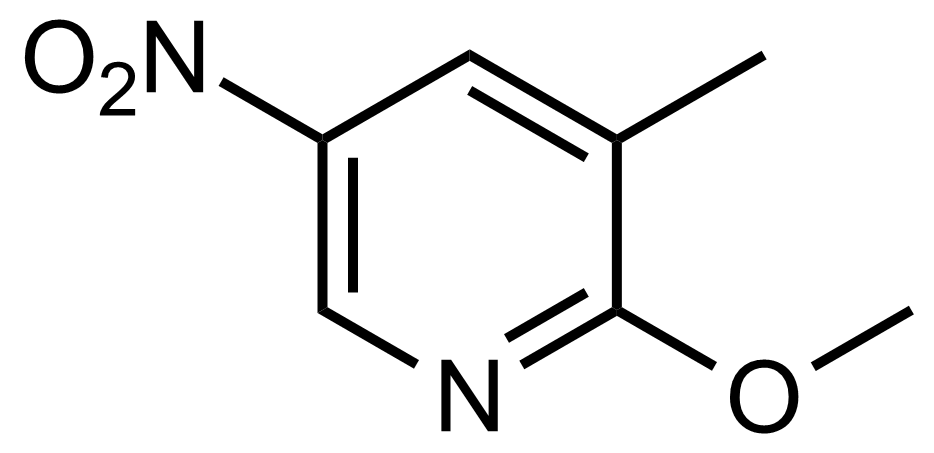
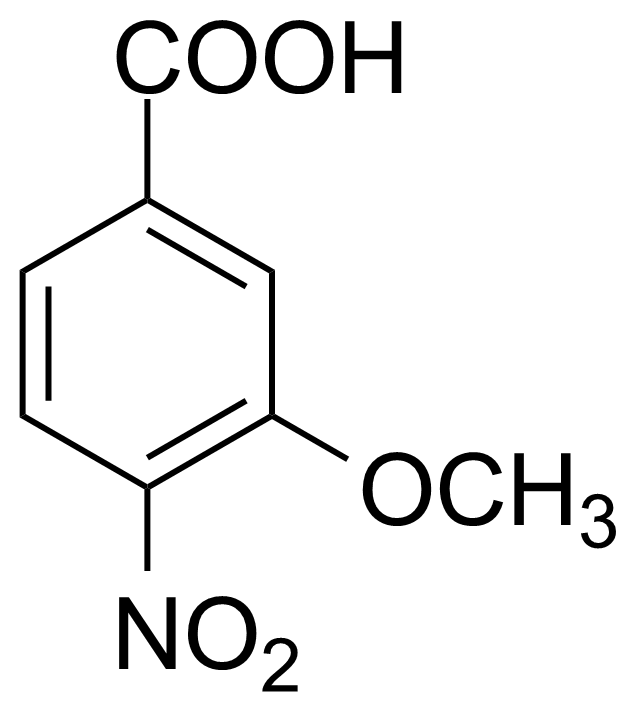

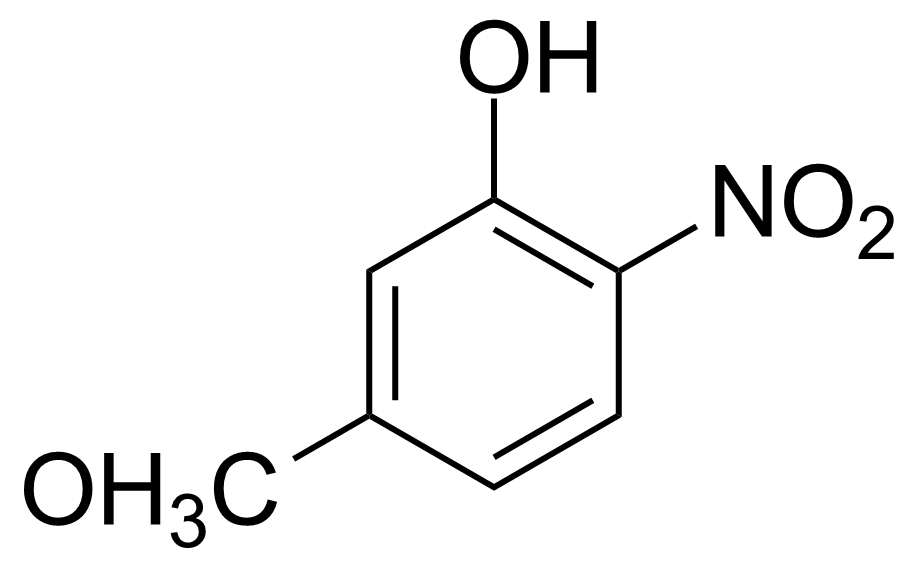
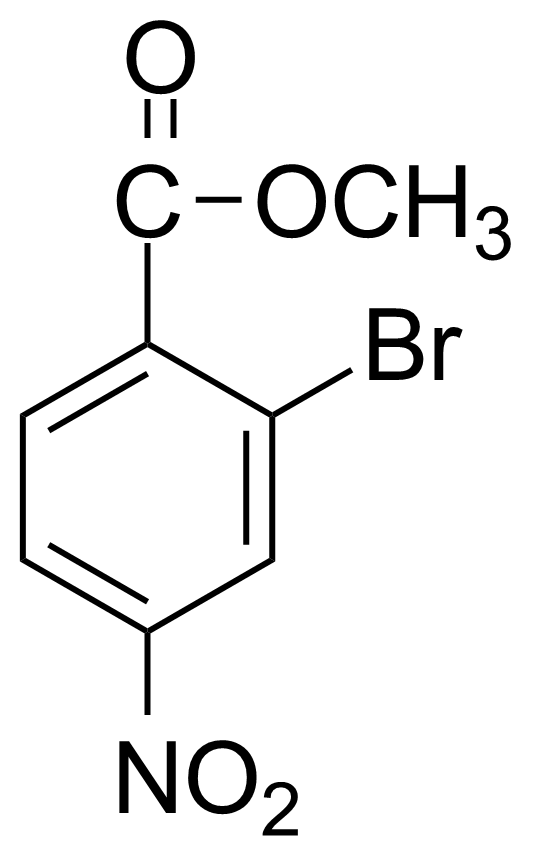
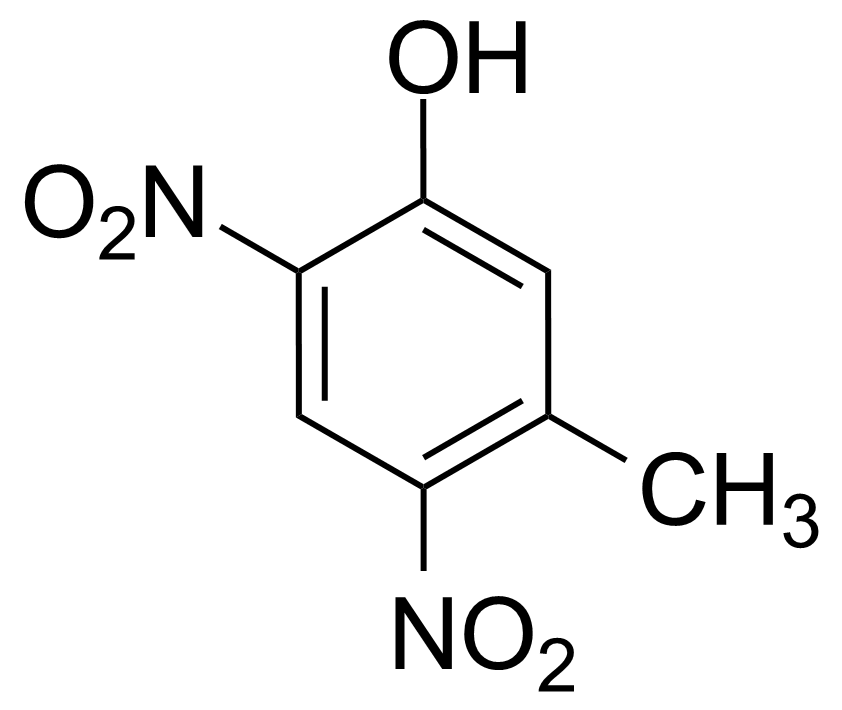
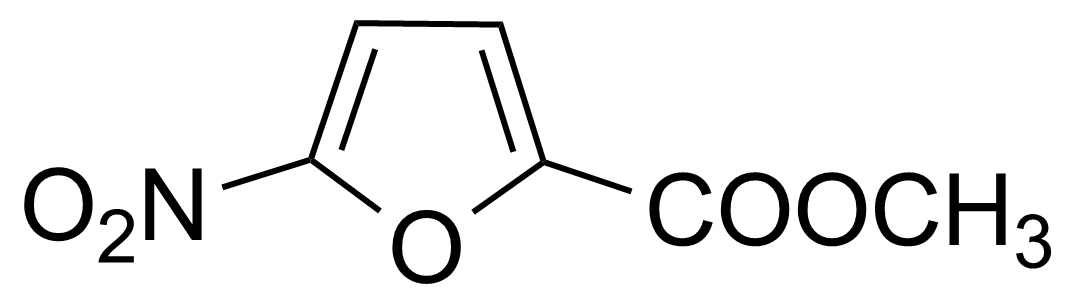
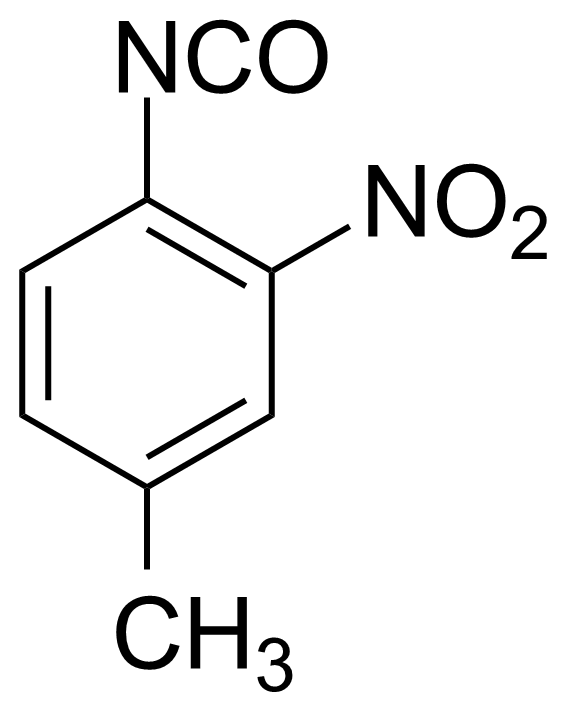
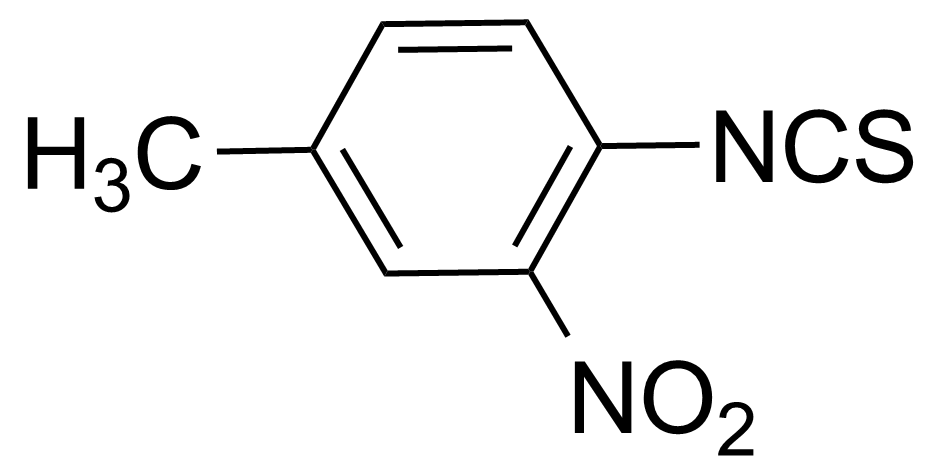
![Structure of Methyl 2'-(5-nitrothiophen-2-yl)-1H,3'H-[2,5'-bibenzo[d]imidazole]-6-carboxylate](https://georganics.sk/wp-content/uploads/2021/06/GEO-01900_Methyl_2-5-nitrothiophen-2-yl-1H3H-25-bibenzodimidazole-6-carboxylate.png)
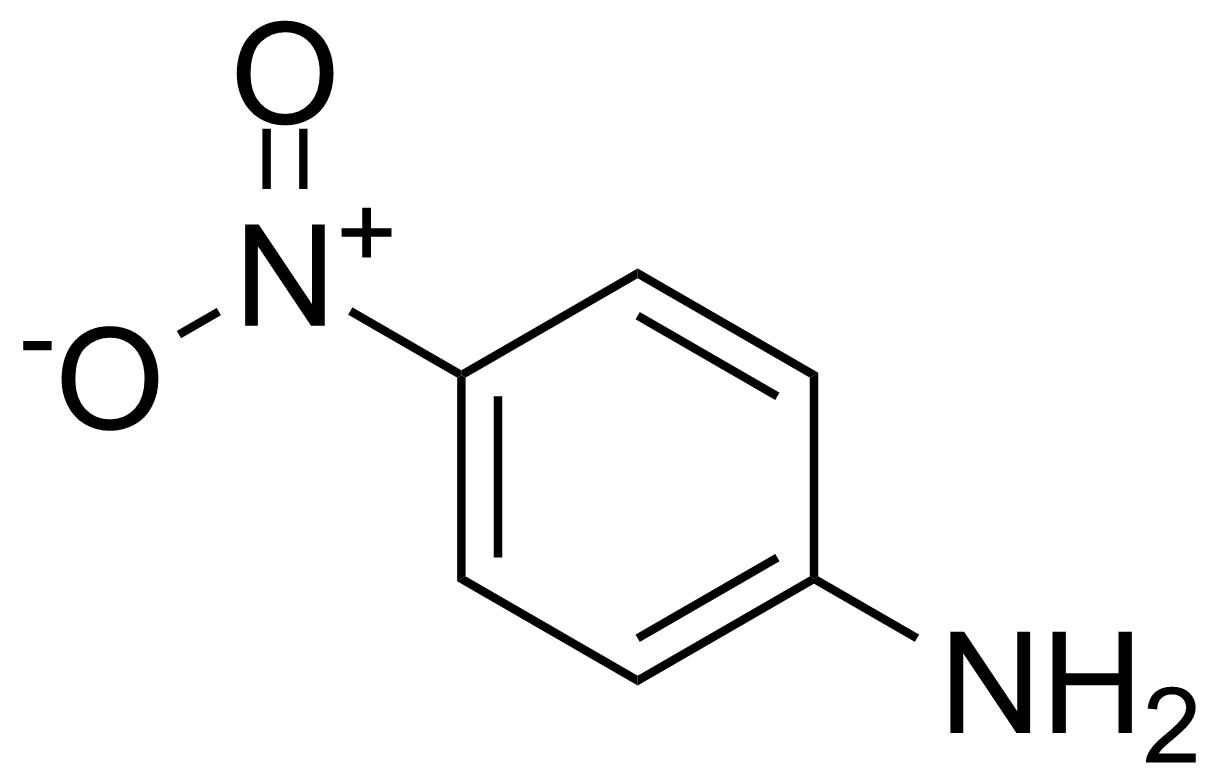
![Structure of 2-(6-Nitro-2,3-dihydrobenzo[b][1,4]dioxin-5-yl)acetonitrile](https://georganics.sk/wp-content/uploads/2021/06/GEO-03680_2-6-Nitro-23-dihydrobenzob14dioxin-5-ylacetonitrile.png)