Isocyanides
Isocyanides are isomers of related nitriles which organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They can be prepared by dehydration of formamides, Hoffmann isocyanide synthesis via dichlorocarbene intermediate or by deprotonation of oxazoles and benzoxazoles in the 2-position. They are stable to strong base but sensitive to acid. In the presence of aqueous acid, isocyanides hydrolyse to the corresponding formamides. Some of them can polymerize in the presence of Lewis or Bronsted acids. They can participate in many multicomponent reactions such as the Ugi reaction and the Passerini reaction and in cycloaddition reactions. Isocyanides form coordination complexes with most transition metals.
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| Benzyl isocyanide | 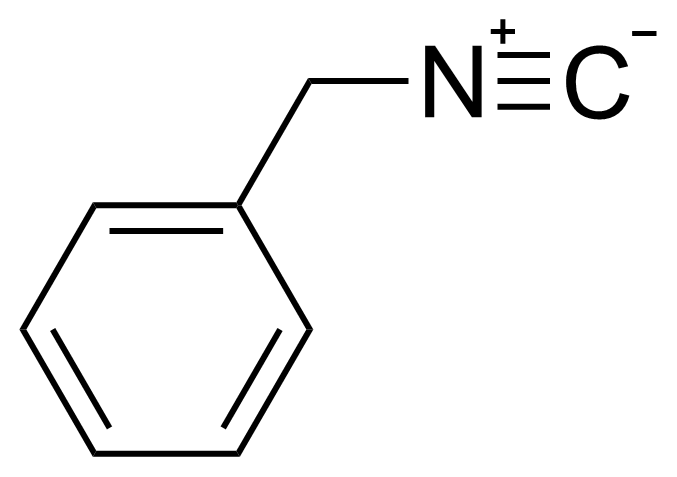 | [10340-91-7] | GEO-02805 | |
| 4-Chlorophenyl isocyanide | 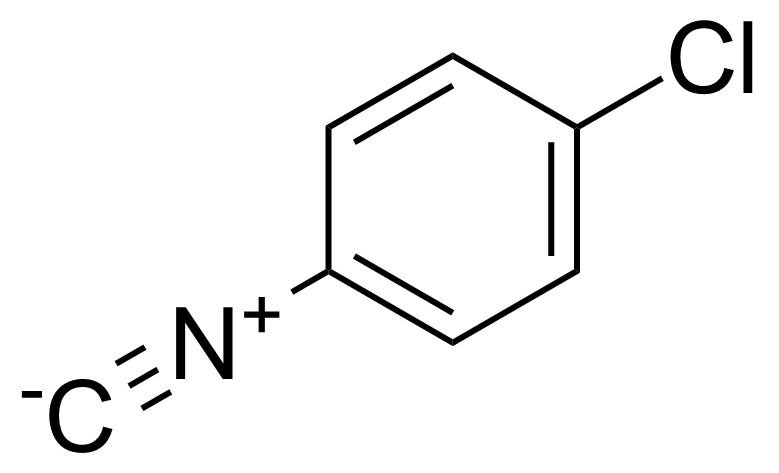 | [1885-81-0] | GEO-03705 | |
| 3-Chlorophenyl isocyanide | 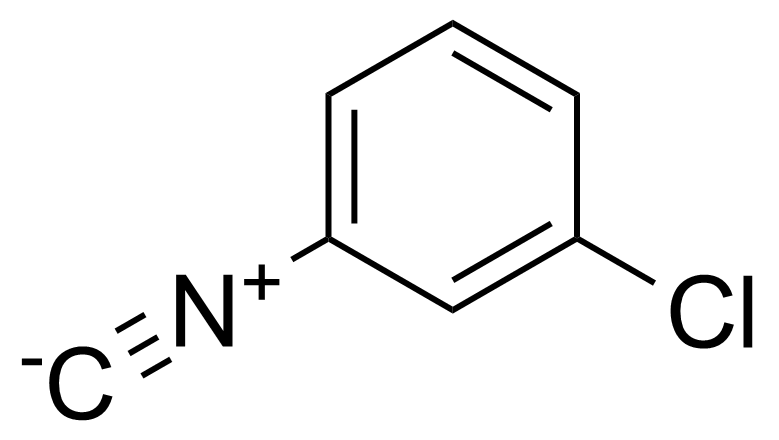 | [32686-54-7] | GEO-03704 | |
| Cyclohexyl isocyanide | 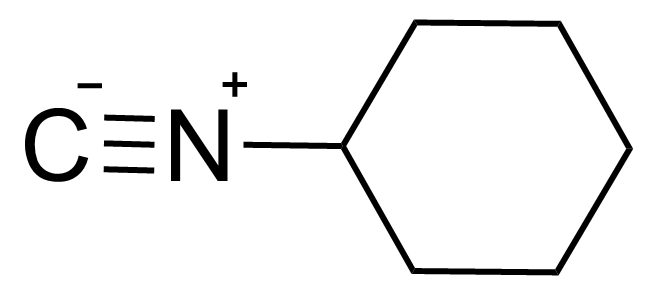 | [931-53-3] | GEO-00871 | |
| Cyclopentylisocyanide | 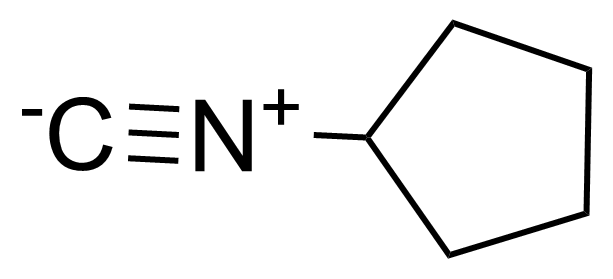 | [68498-54-4] | GEO-02903 | |
| 3,5-Difluoro-1-(isocyanomethyl)benzene | 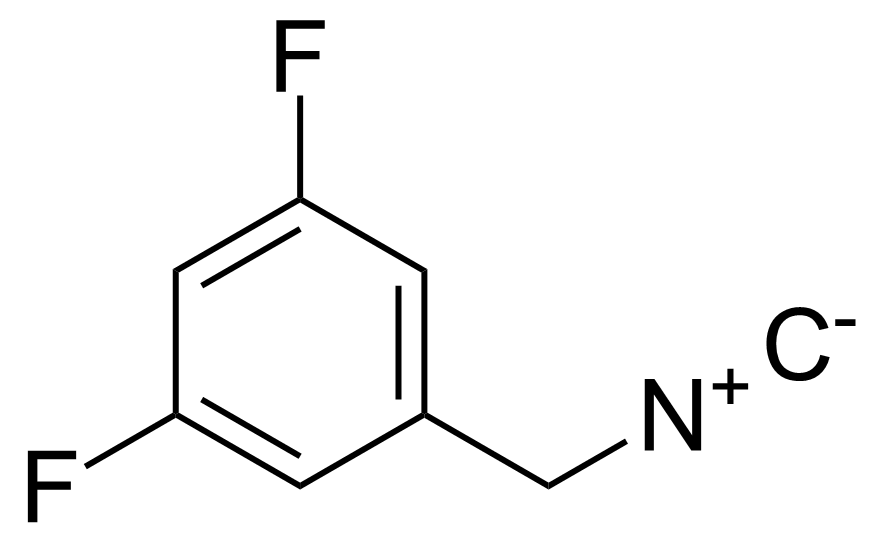 | N/A | GEO-03696 | |
| 2,4-Difluoro-1-(isocyanomethyl)benzene | 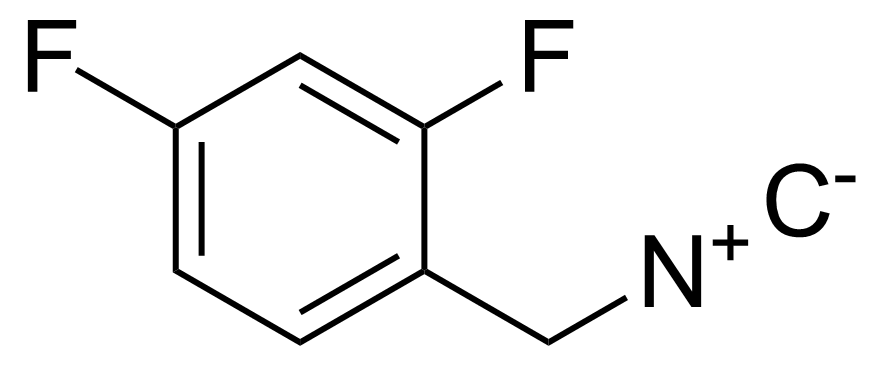 | [730964-55-3] | GEO-03698 | |
| (S)-(-)-alpha-Methylbenzyl isocyanide |  | [21872-32-2] | GEO-03775 | |
| a-Methylbenzyl isocyanide | 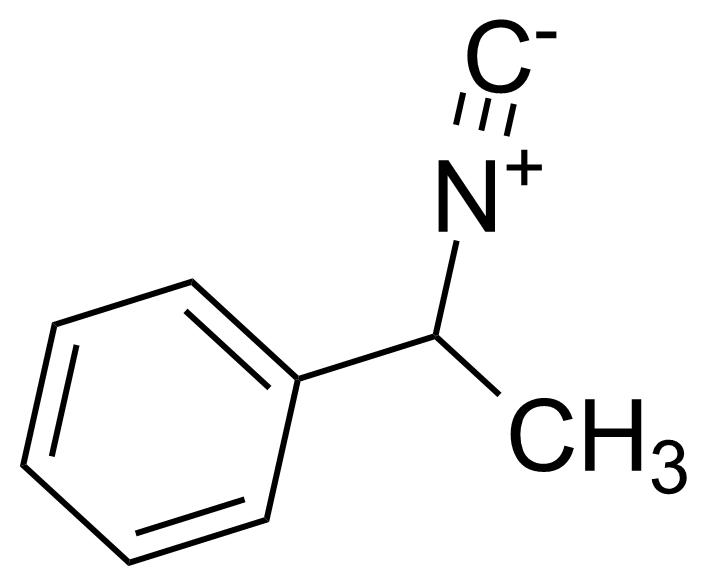 | [17329-20-3] | GEO-01780 | |
| Methyl 2-isocyanoacetate | 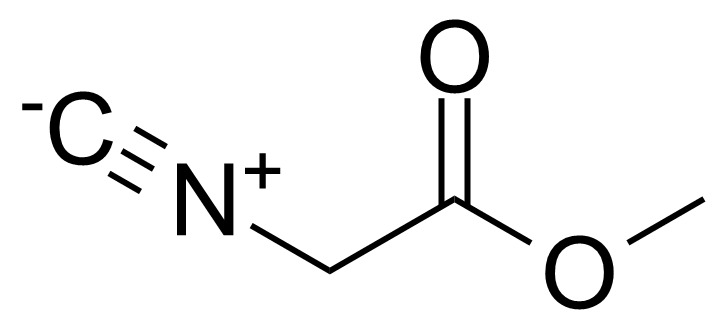 | [39687-95-1] | GEO-03019 | |
| 1,4-Phenylene diisocyanide | 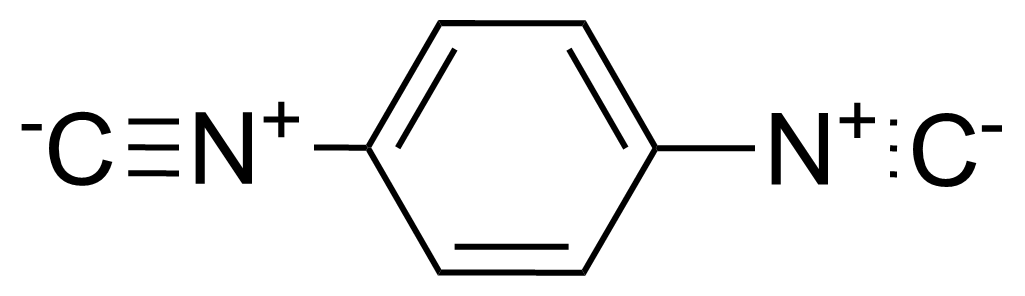 | [935-16-0] | GEO-02803 | |
| Phenylethyl isocyanide | 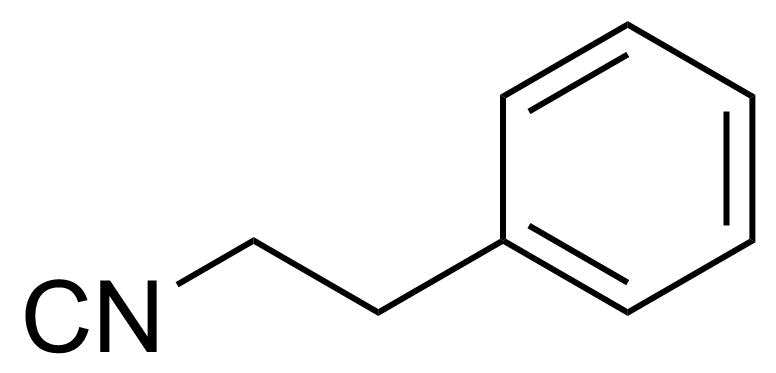 | [59795-89-0] | GEO-03927 | |
| Phenyl isocyanide | 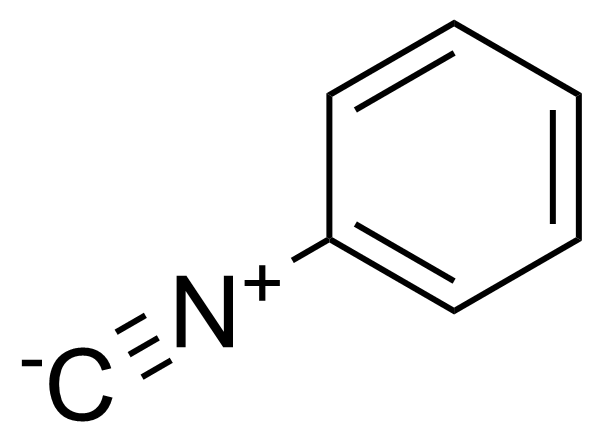 | [931-54-4] | GEO-03710 | |
| 1,1,3,3-Tetramethylbutyl isocyanide | 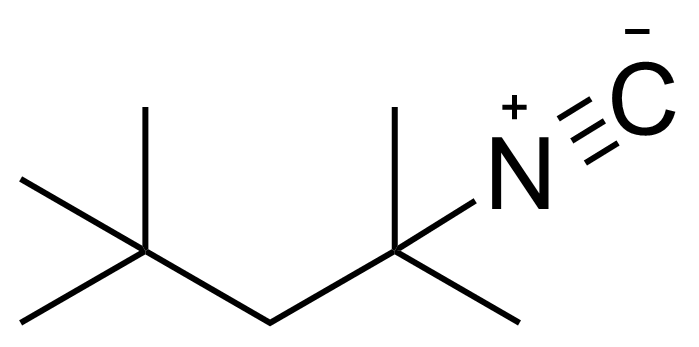 | [14542-93-9] | GEO-02820 |