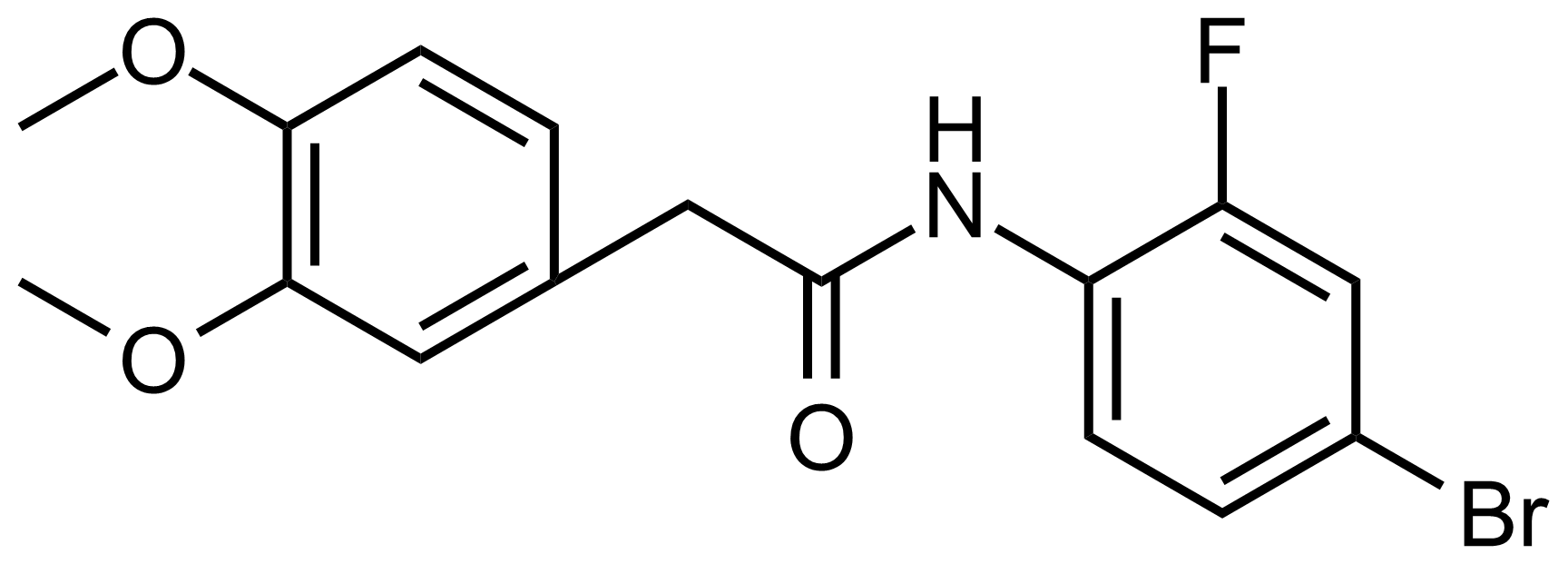
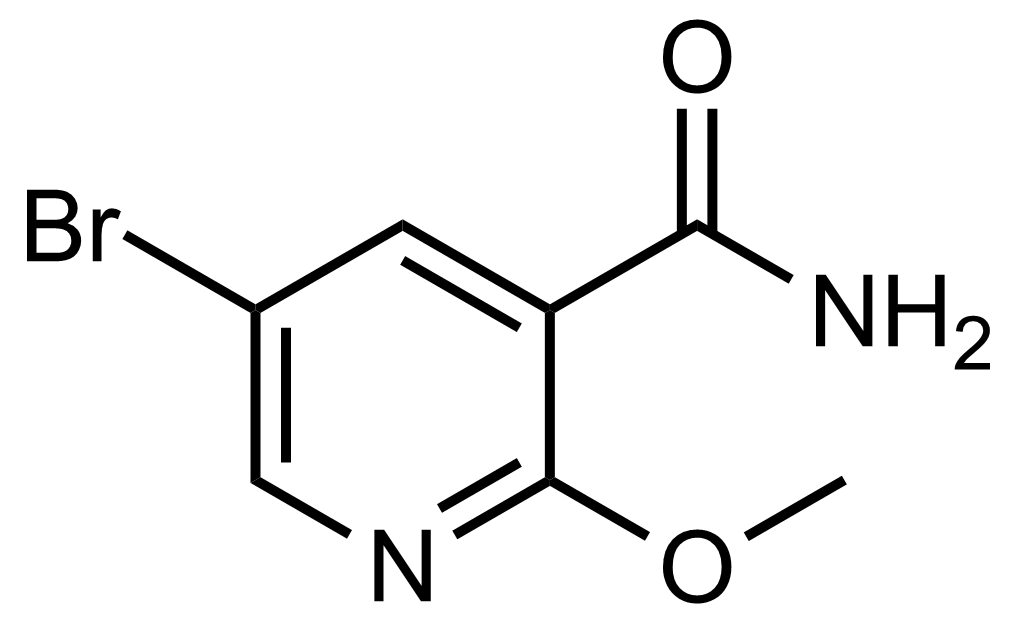
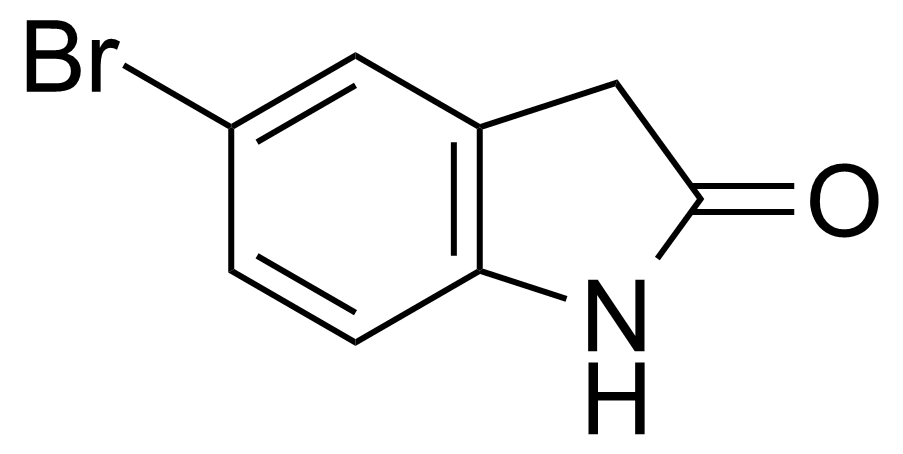
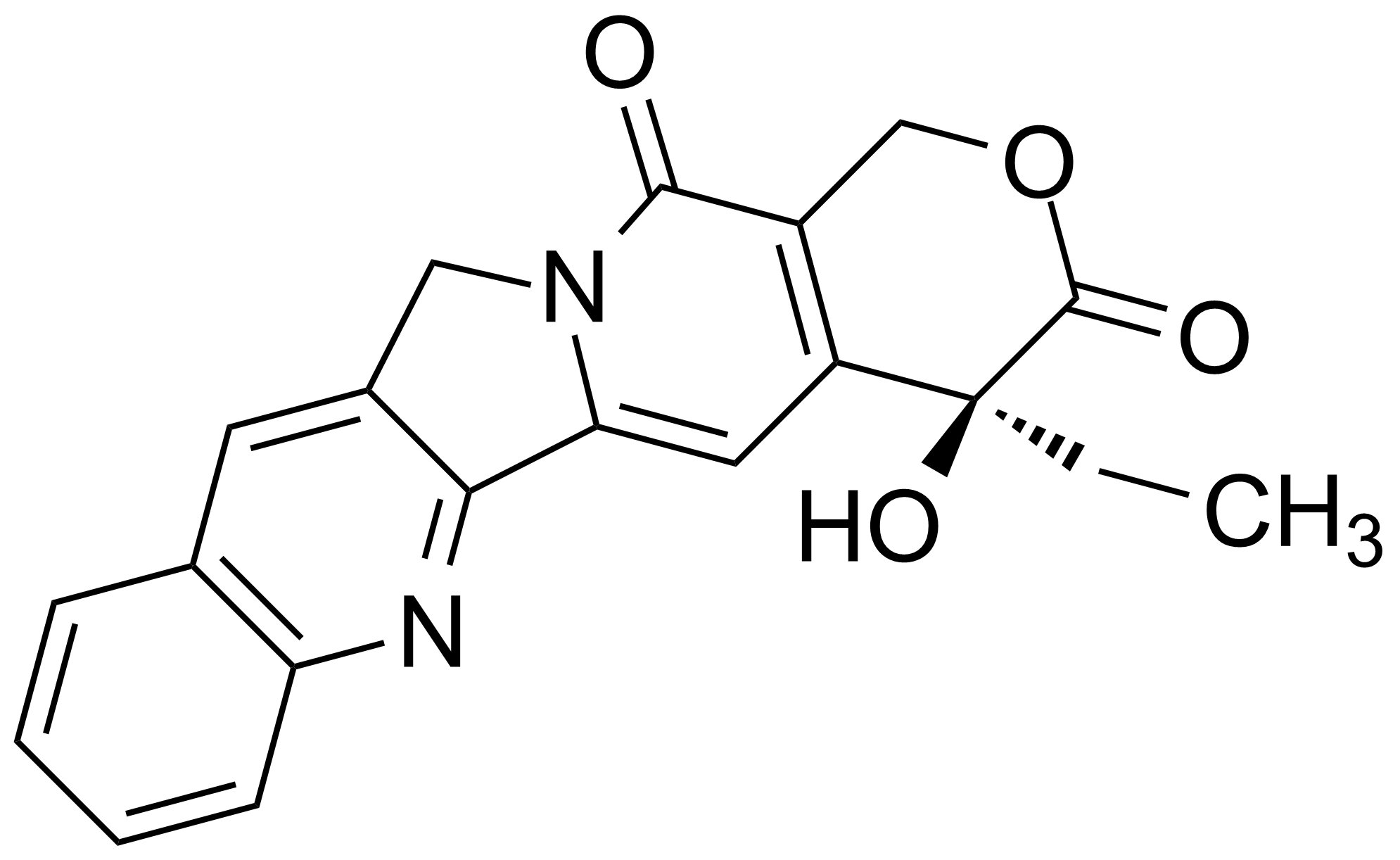
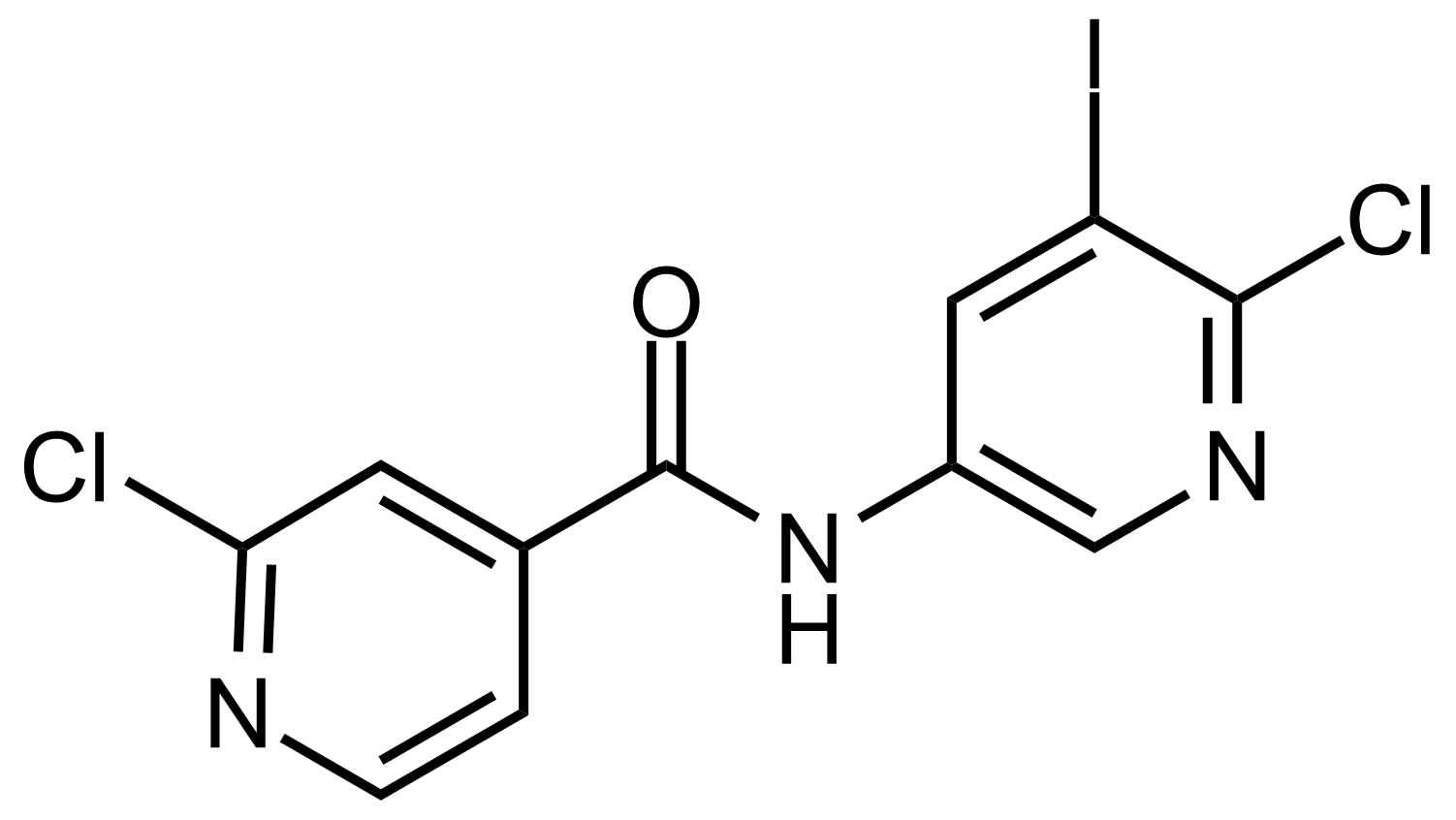
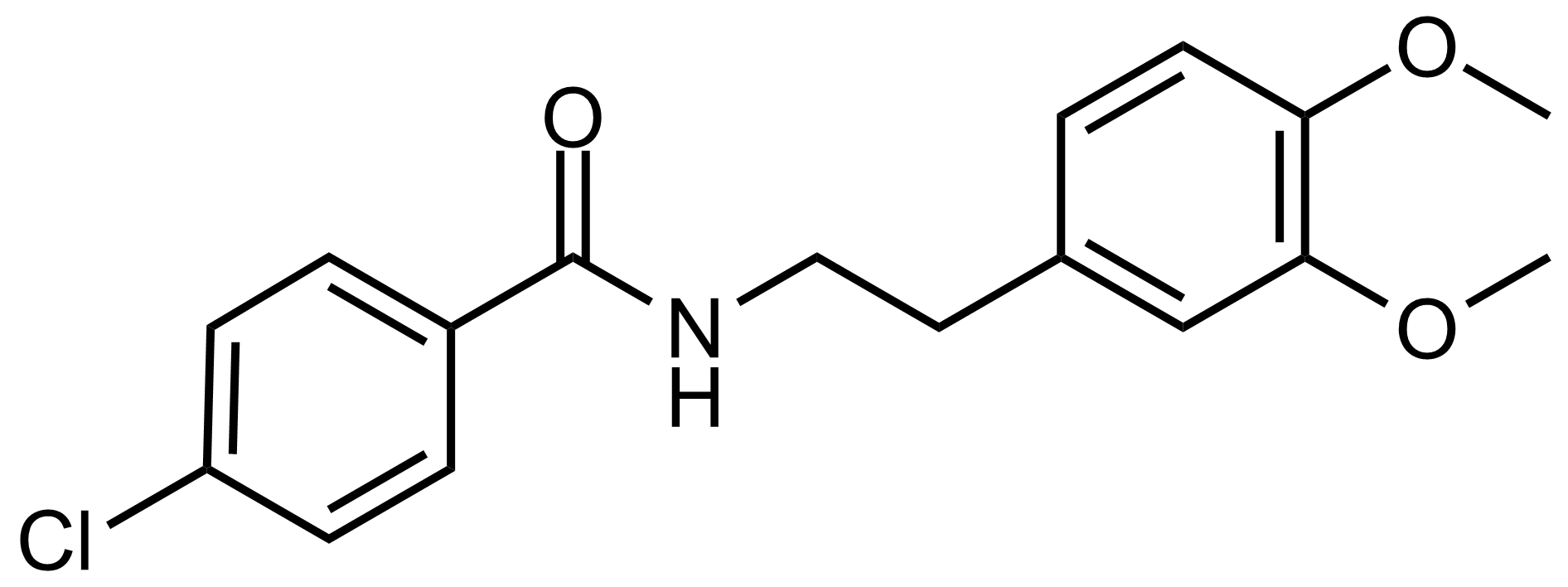
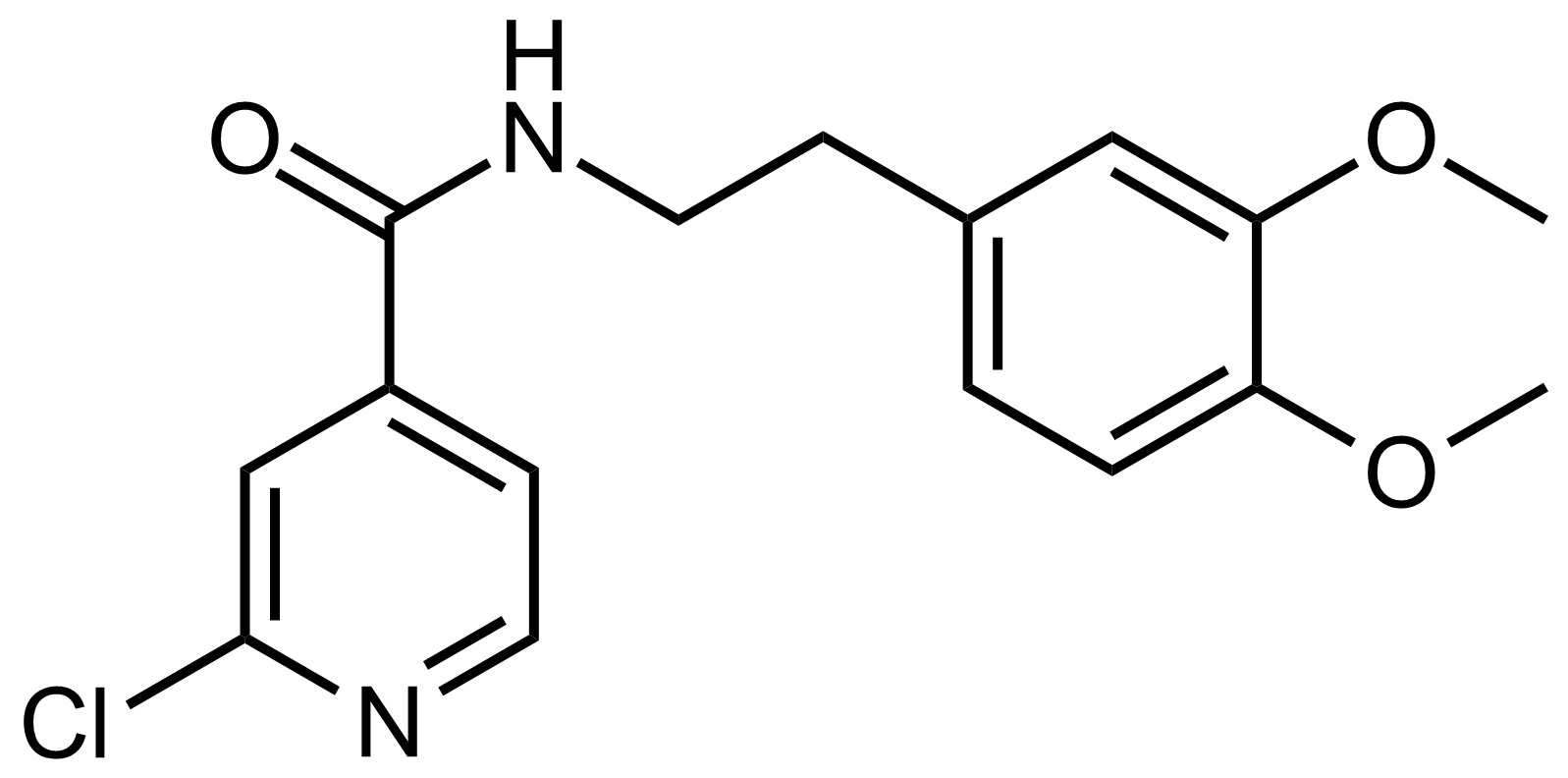
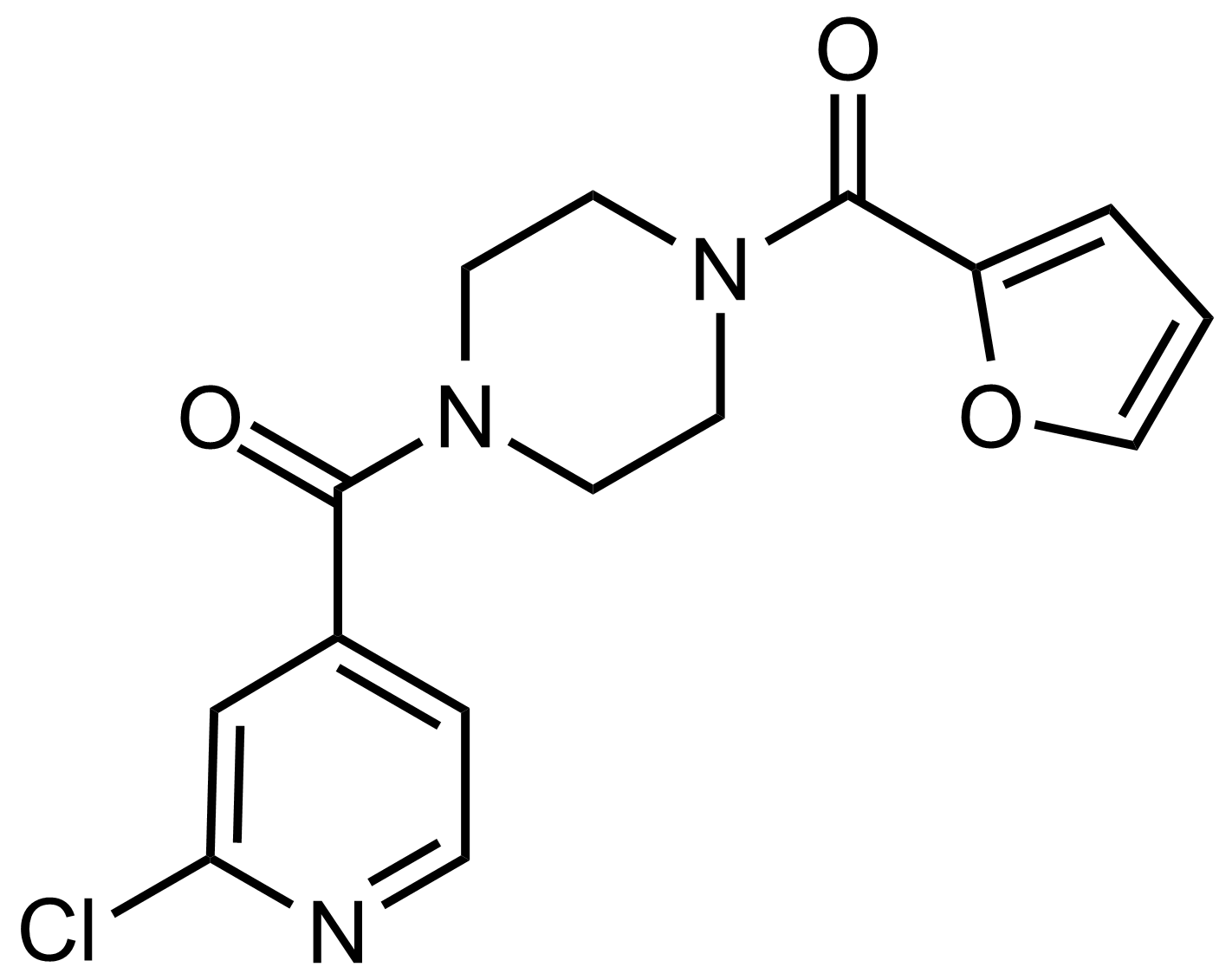
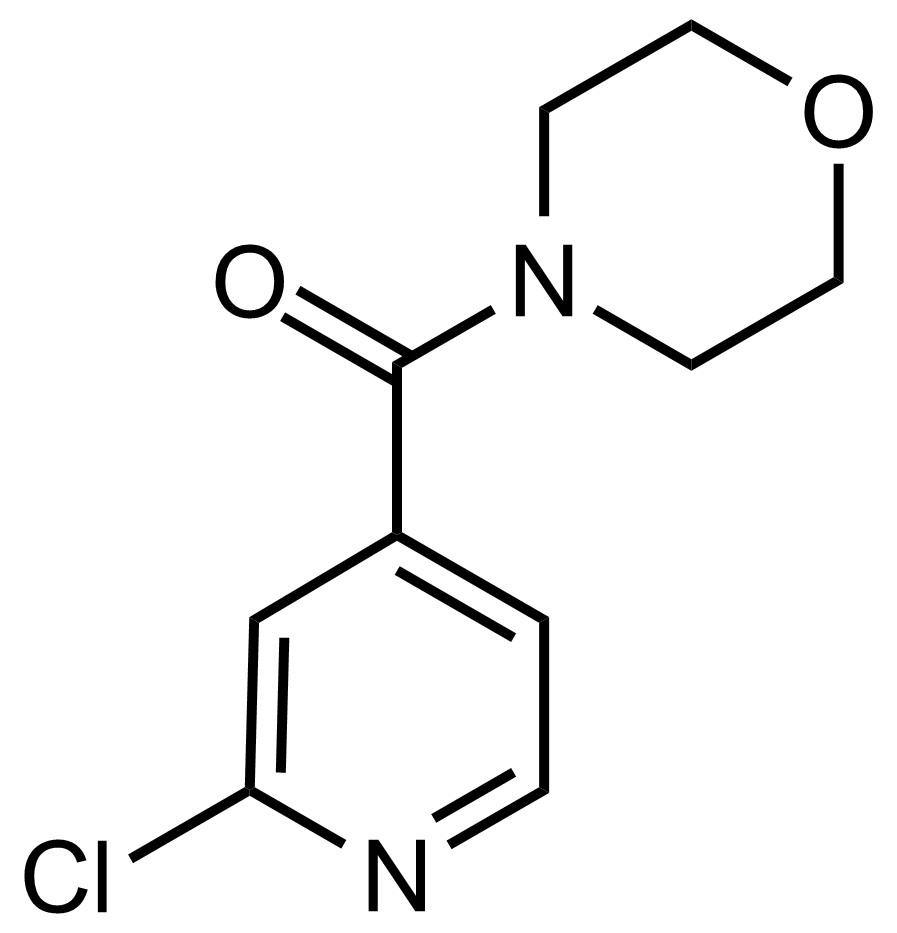
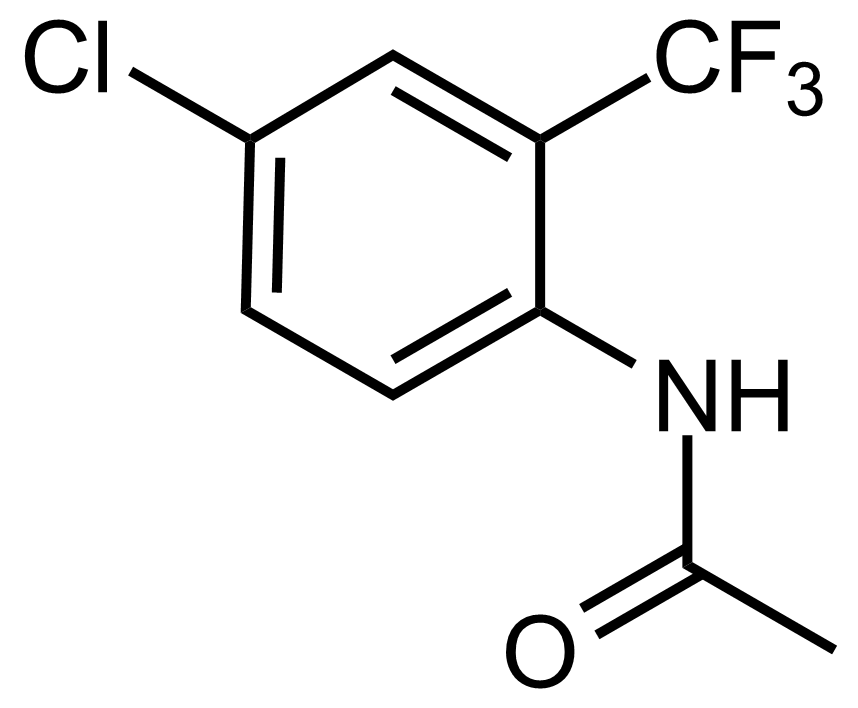
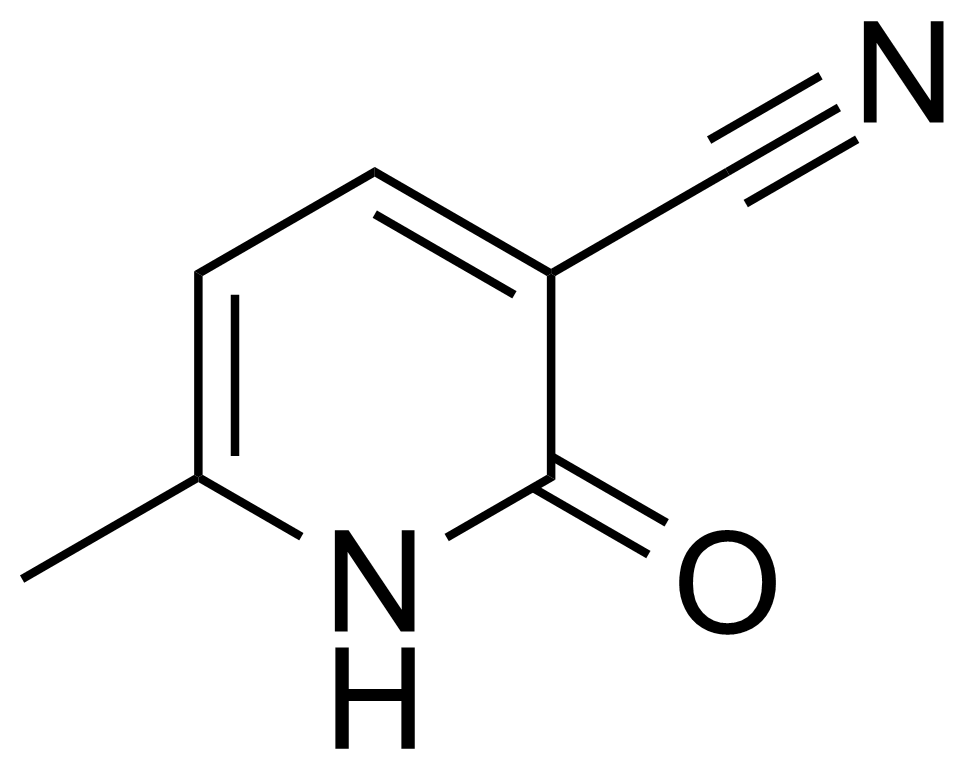
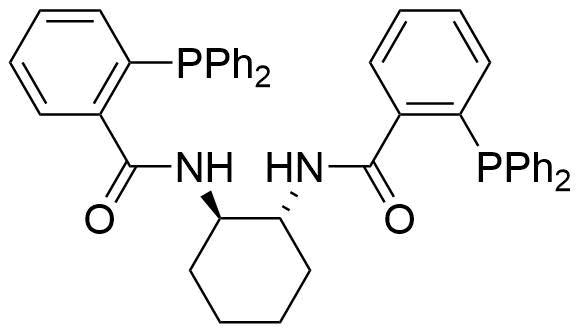
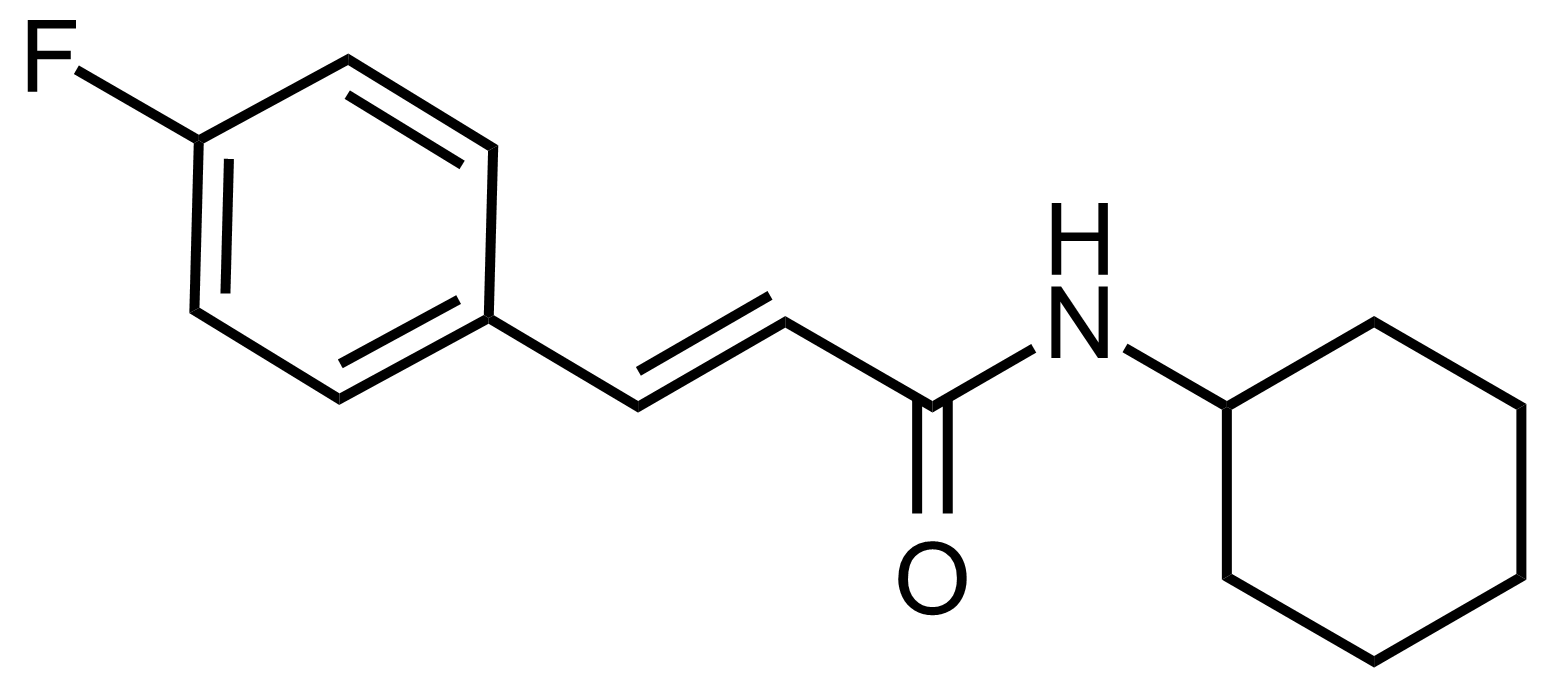
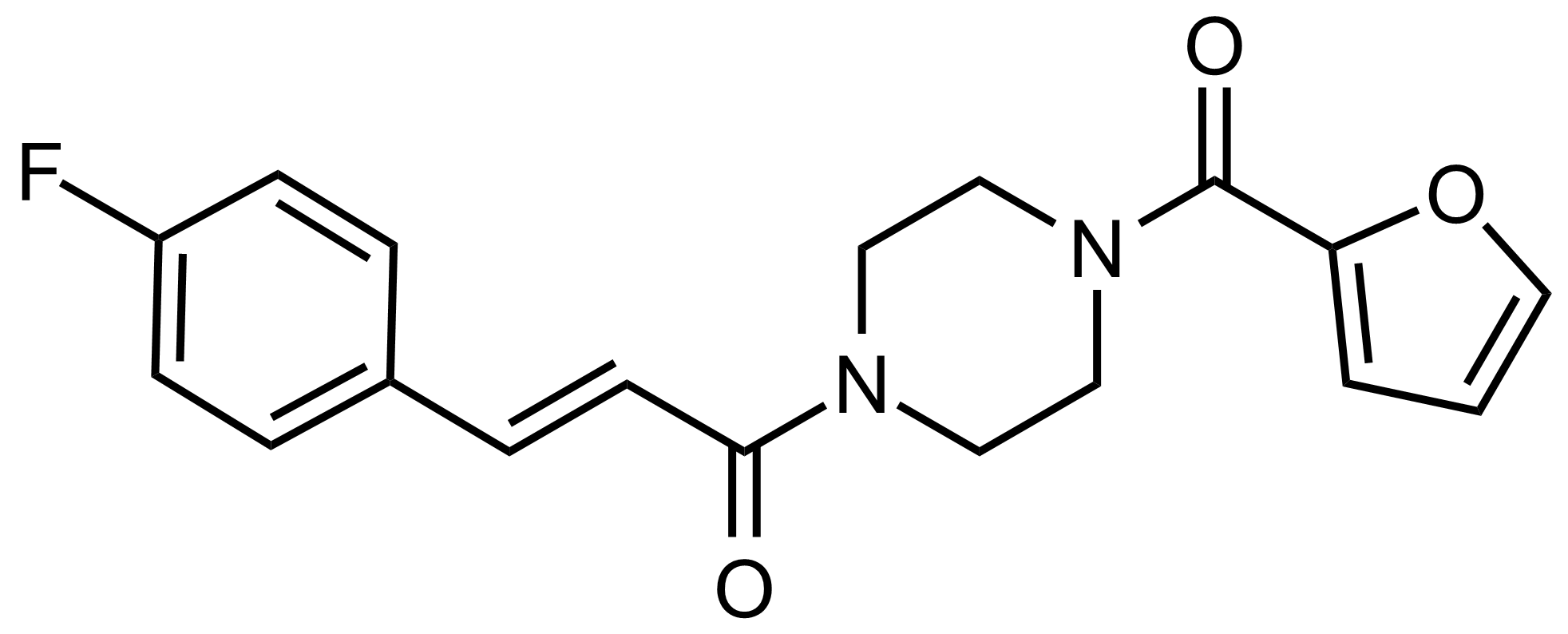
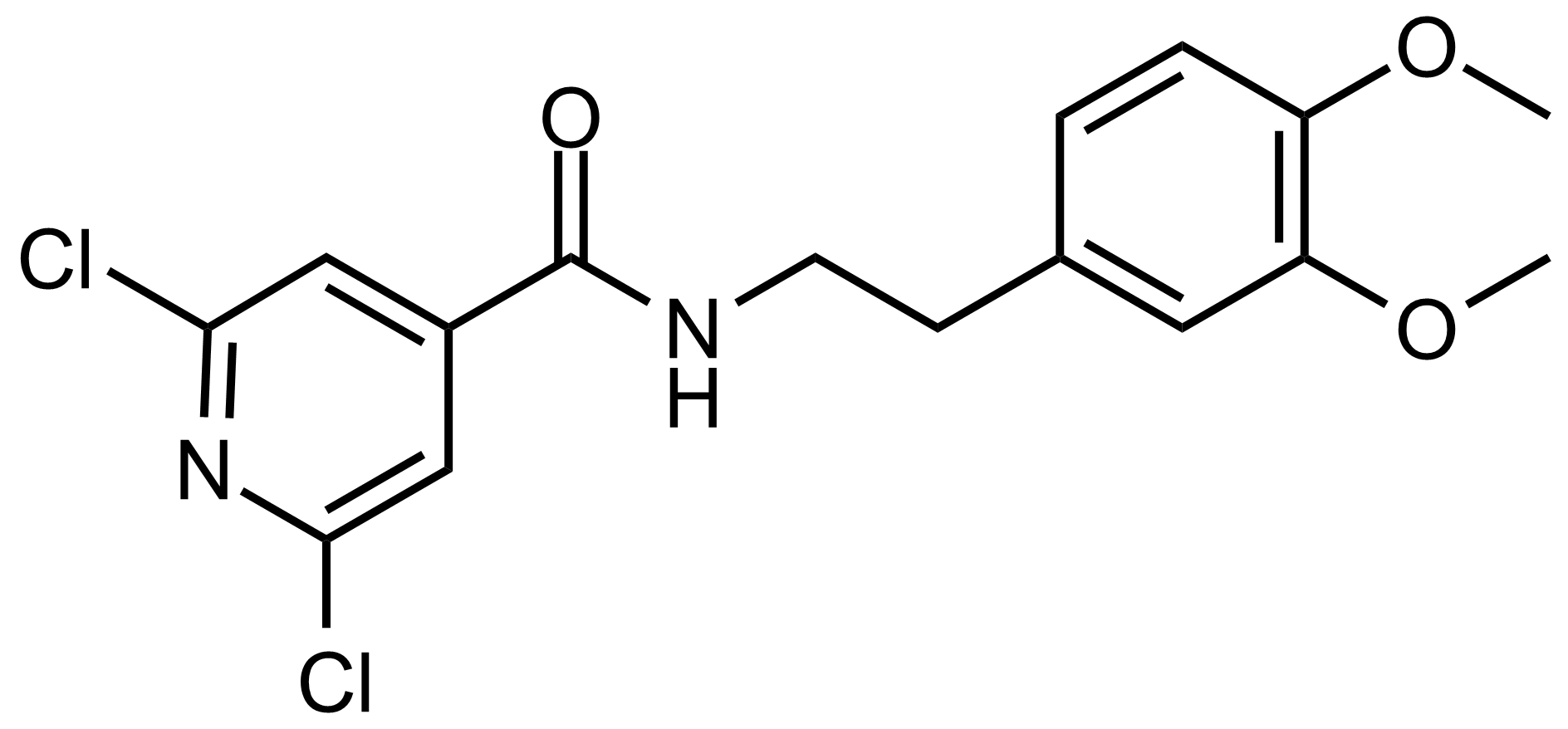
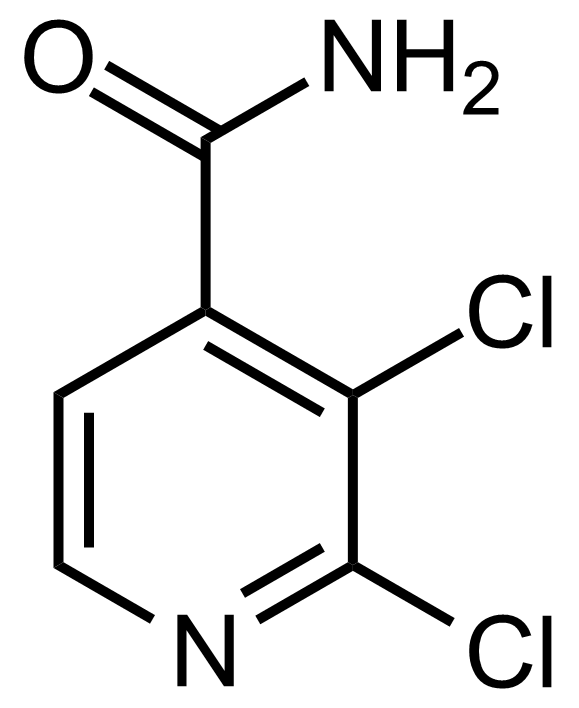
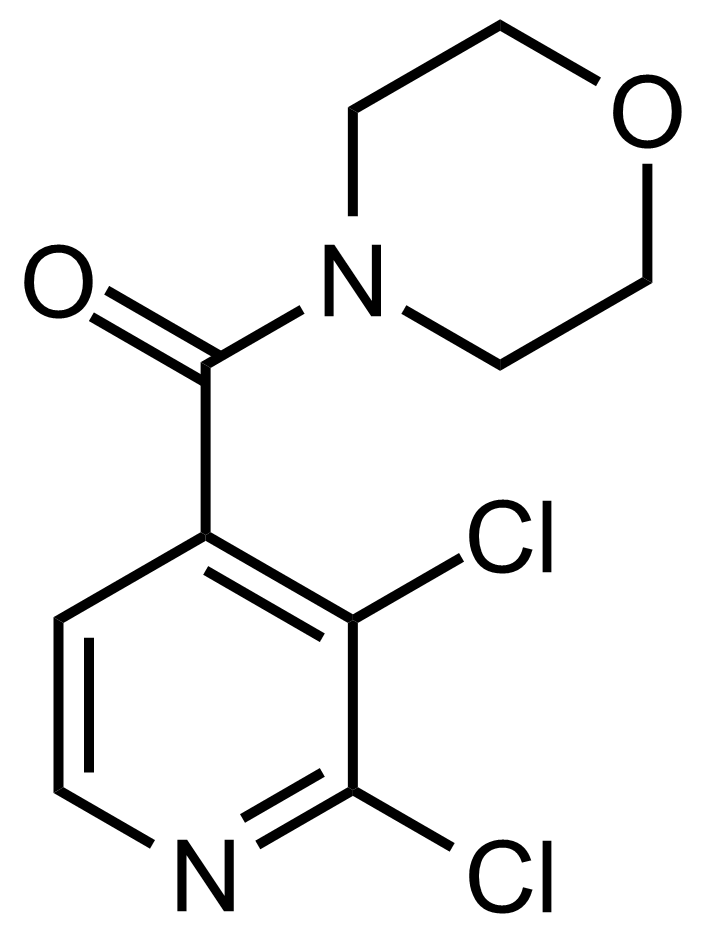
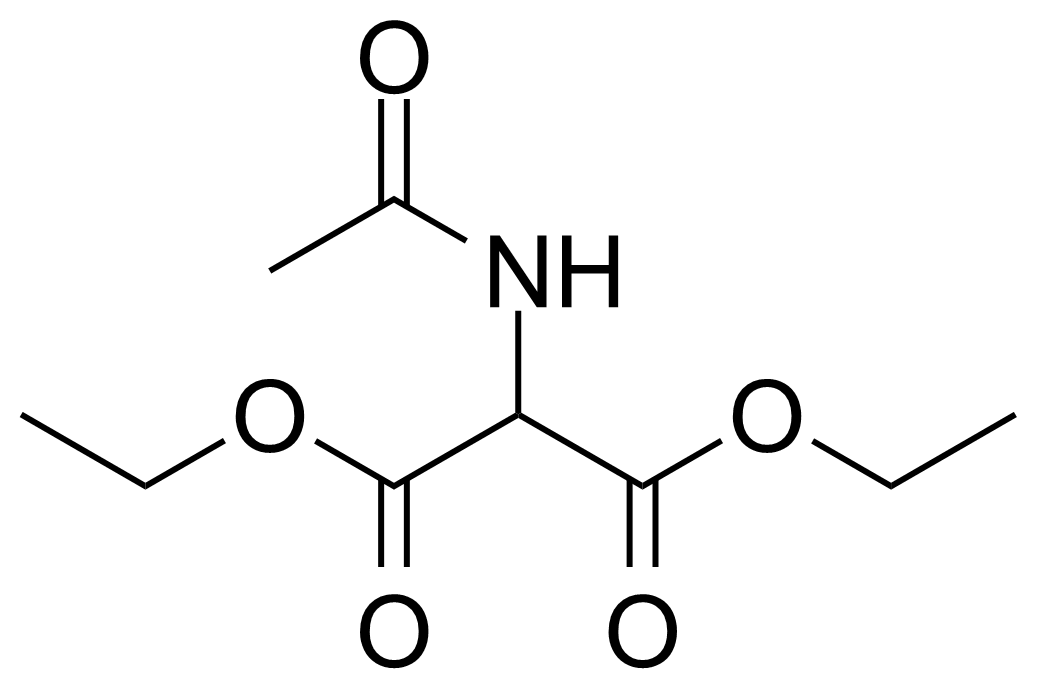
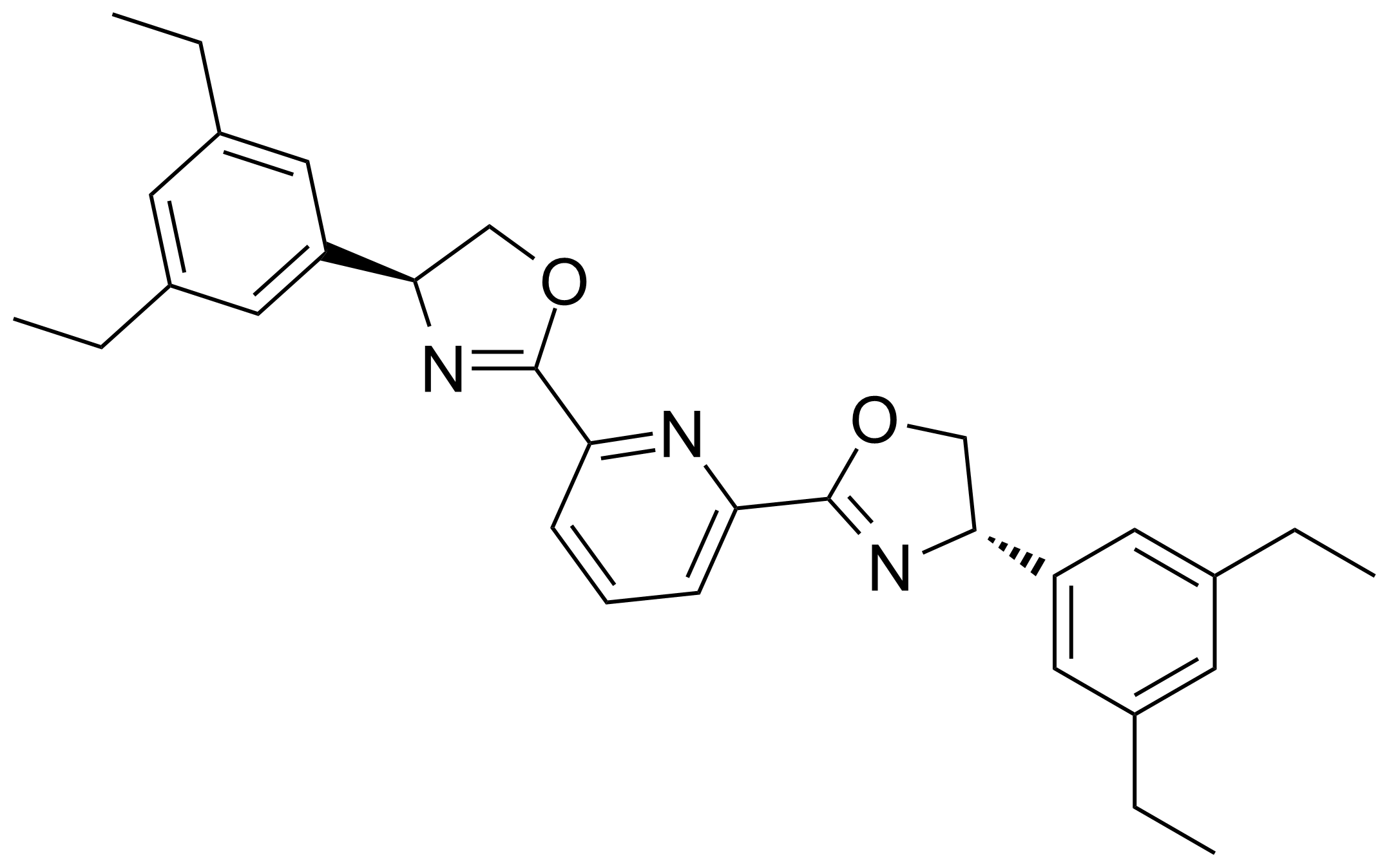
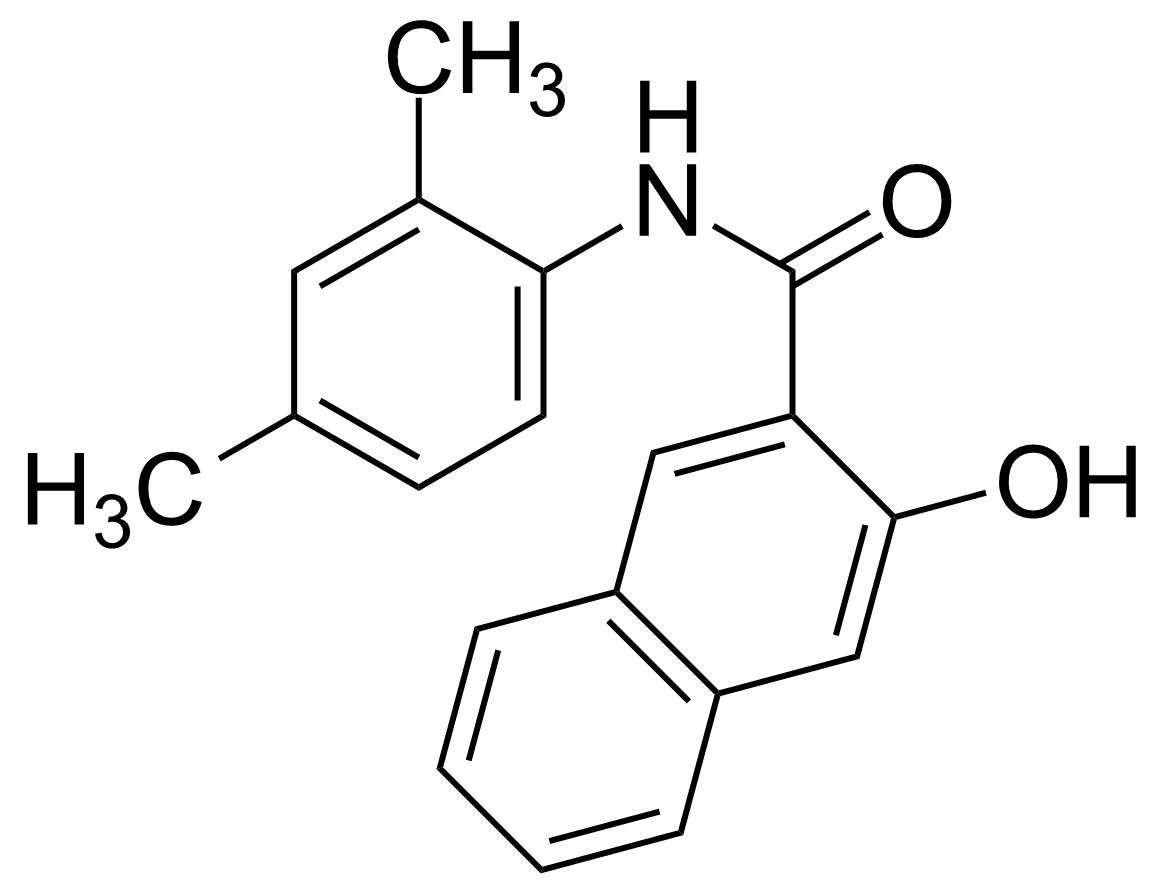
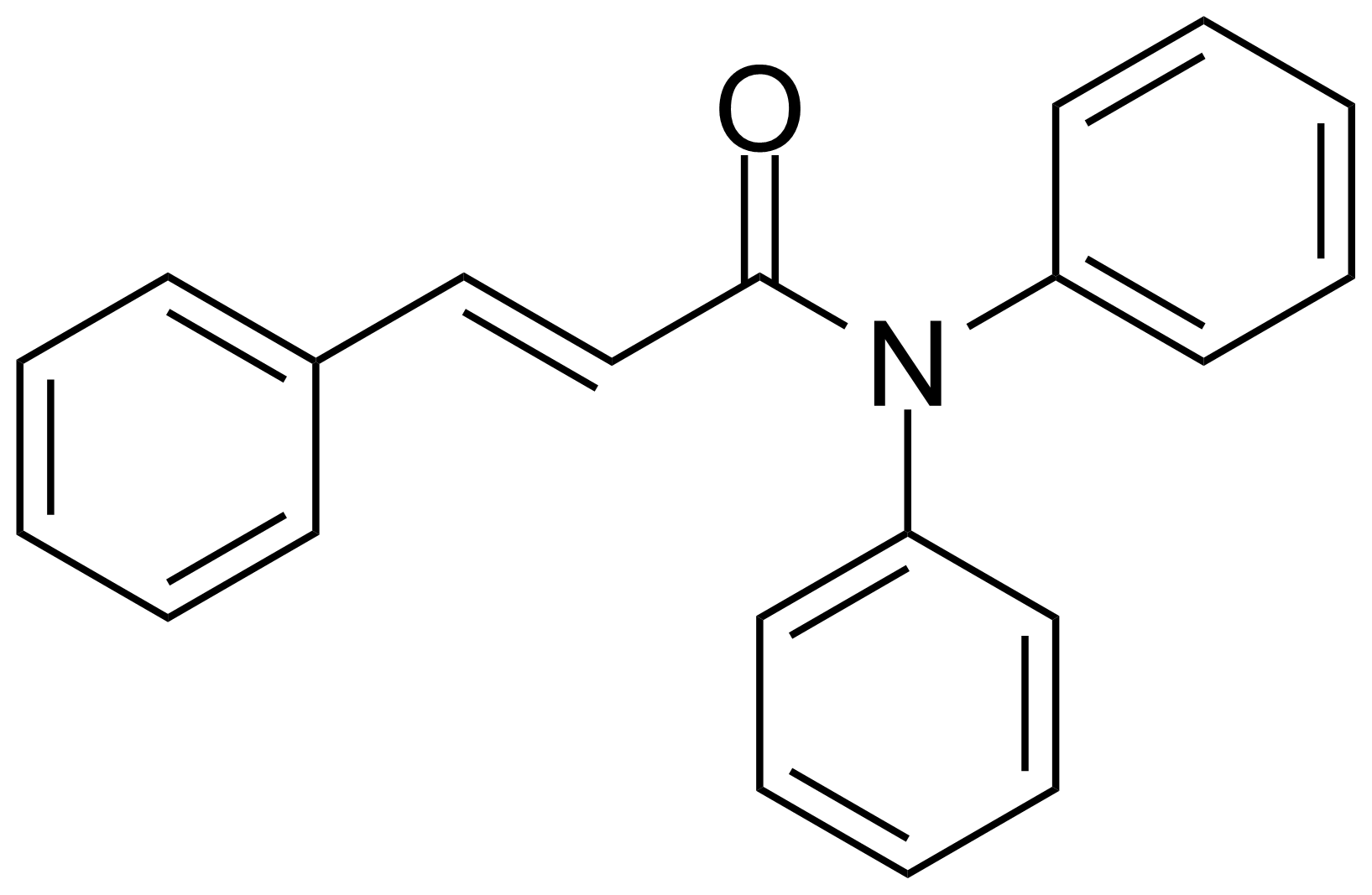
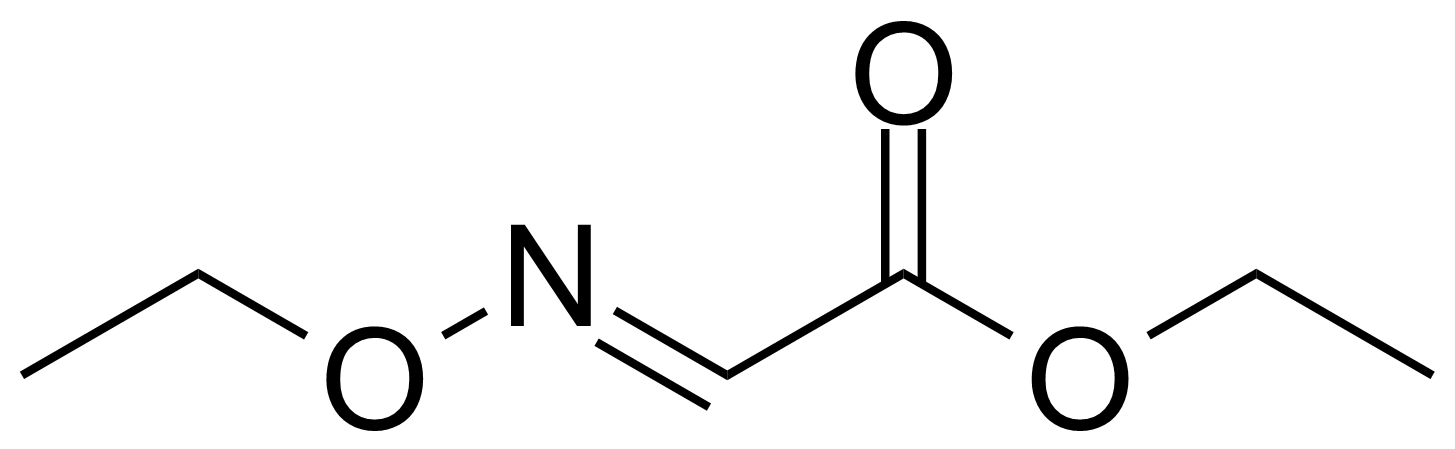
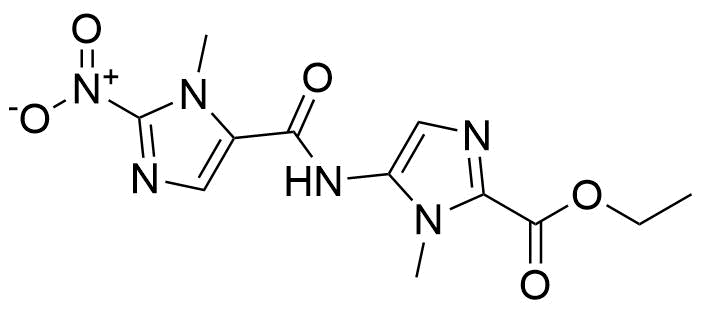
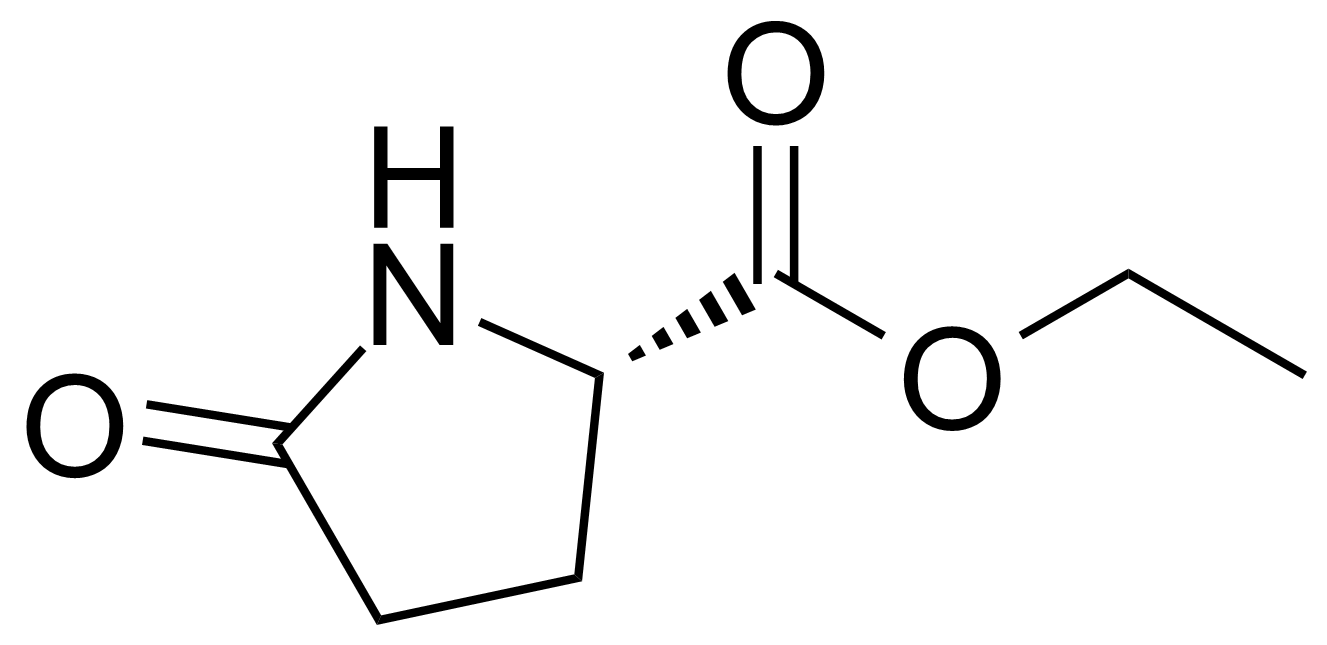
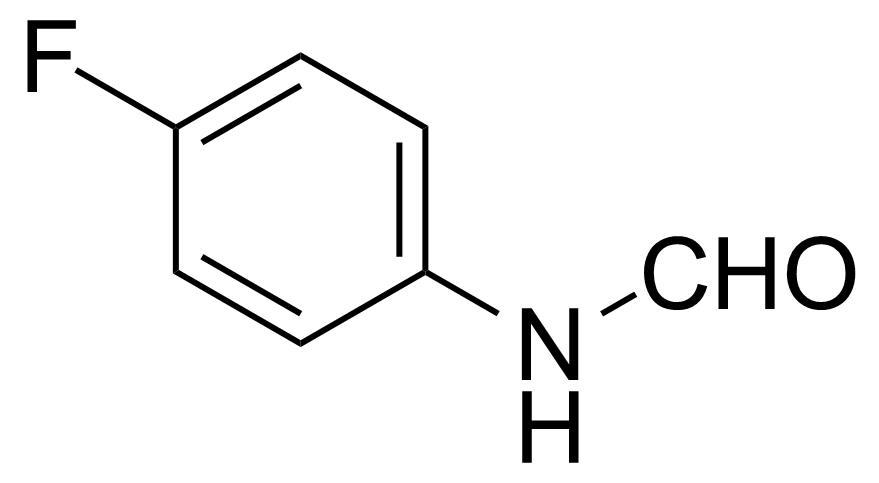
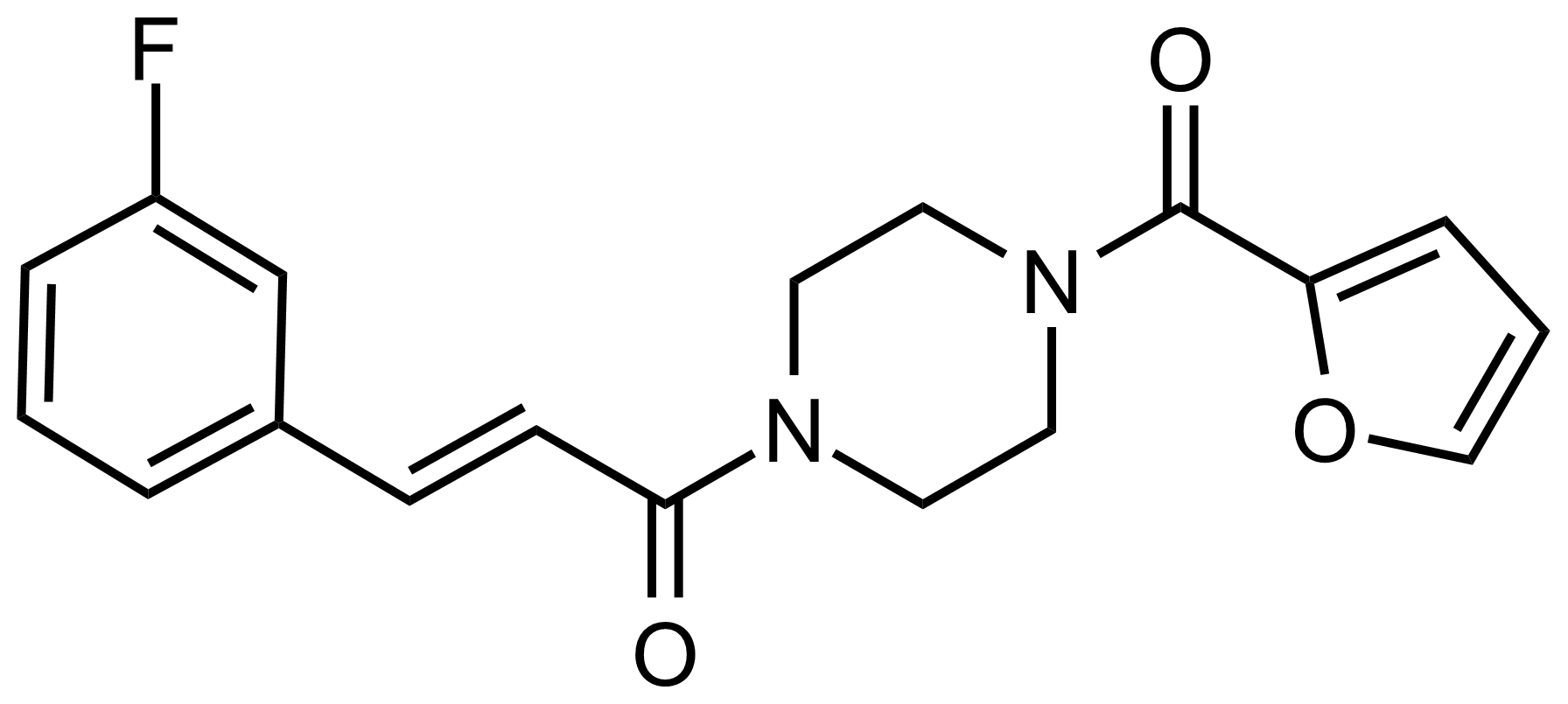
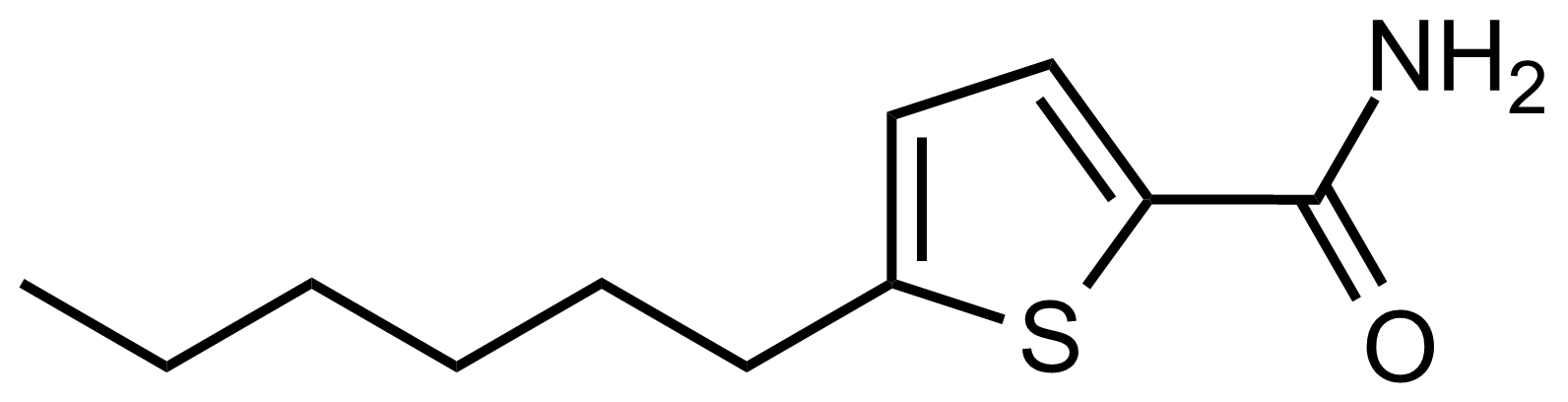
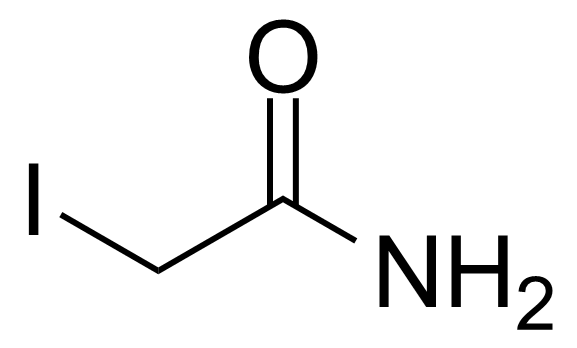
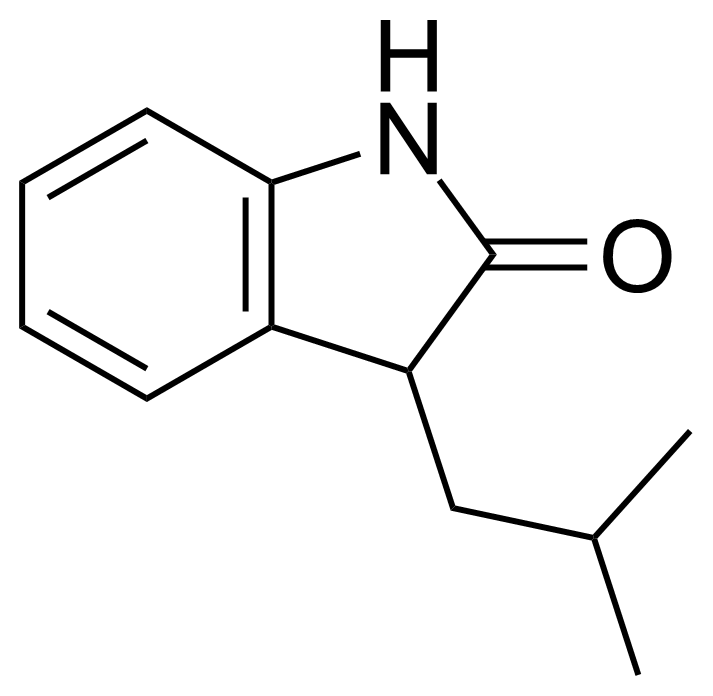
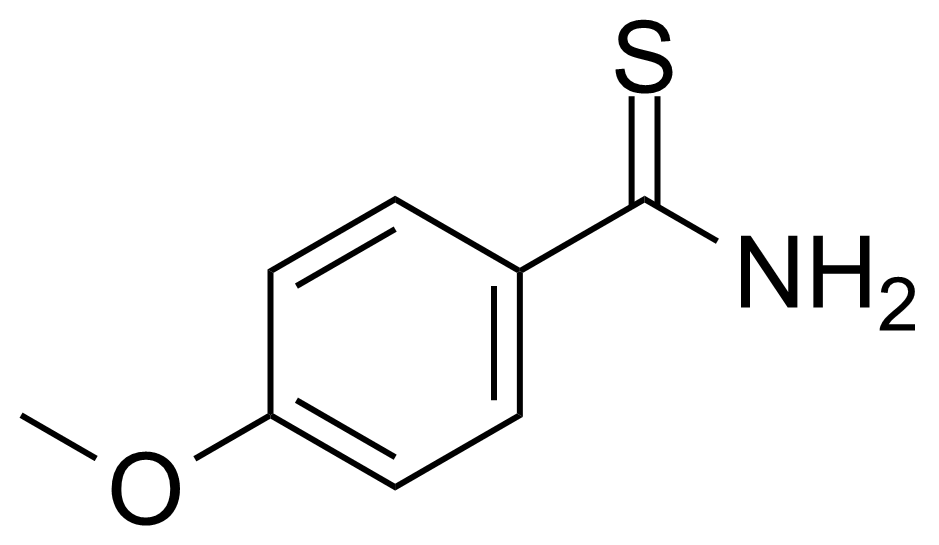
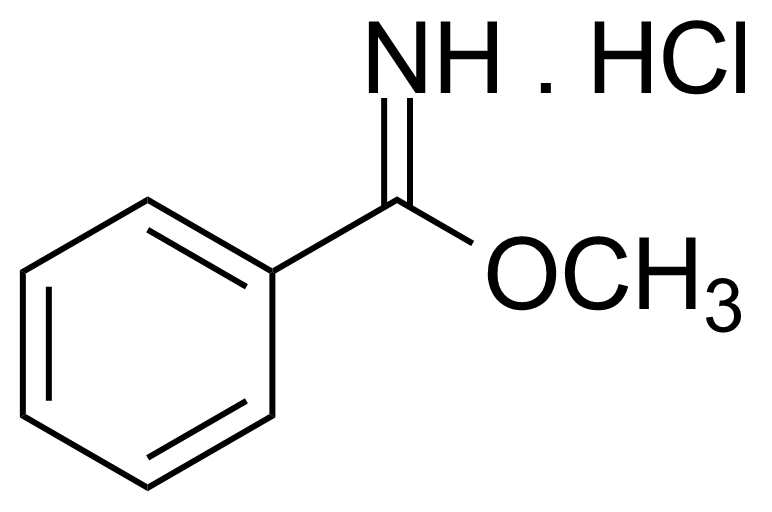
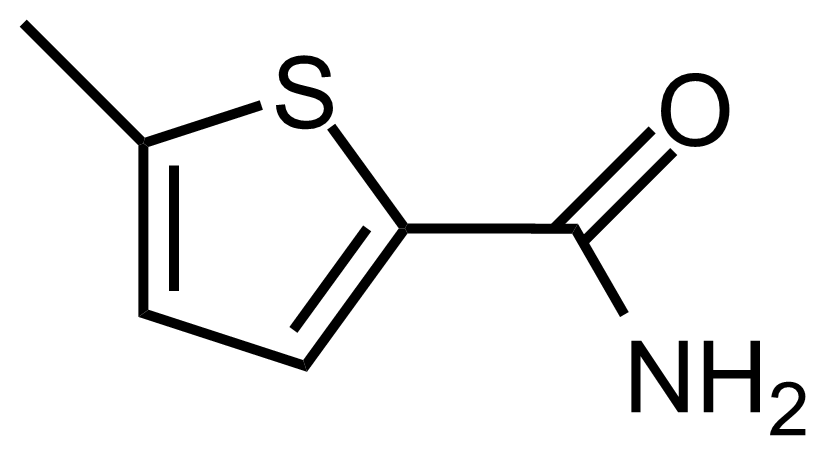
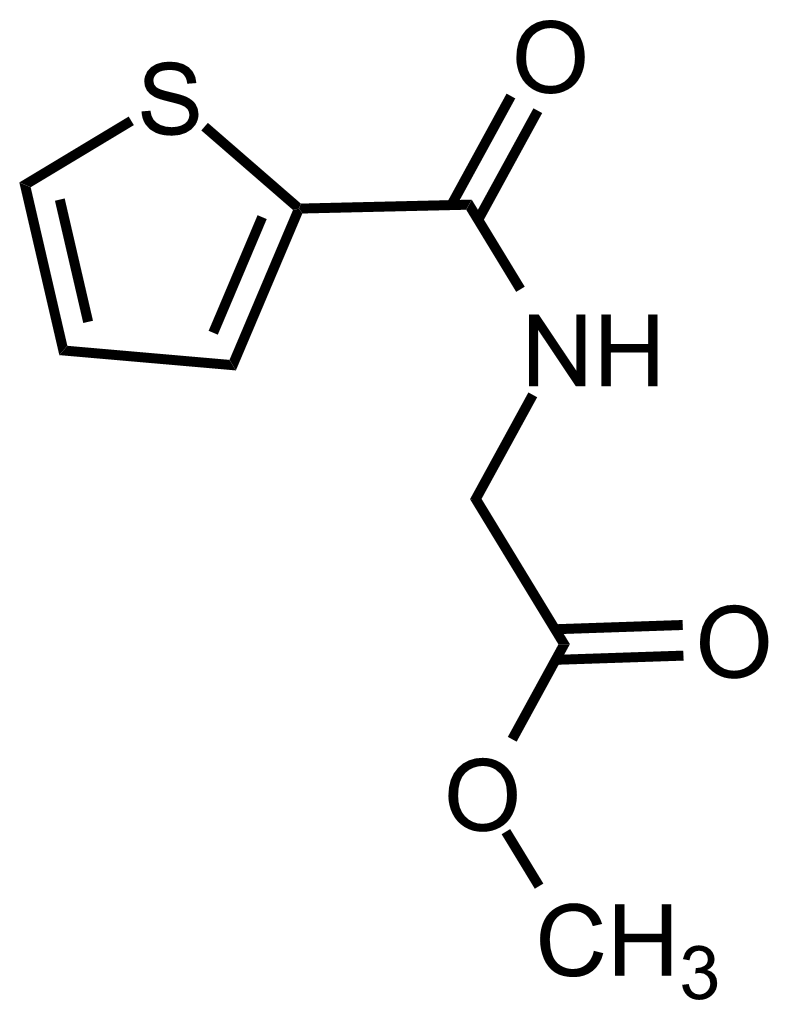
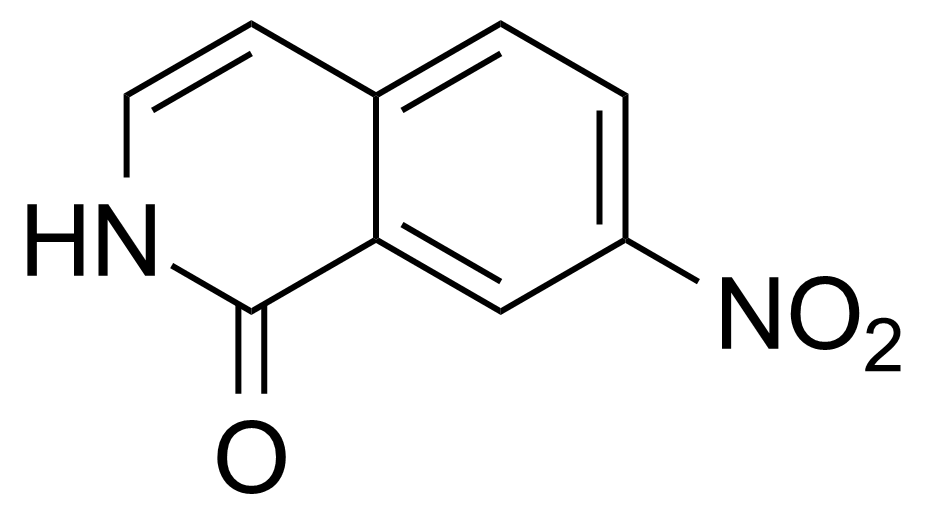
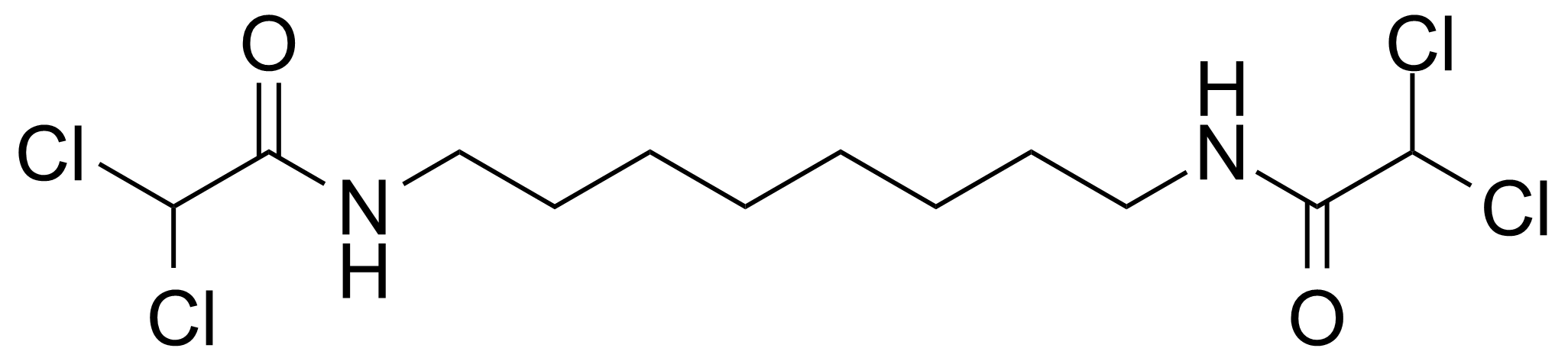
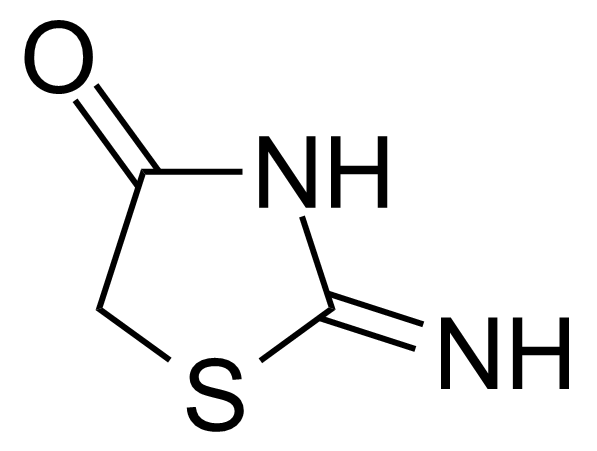
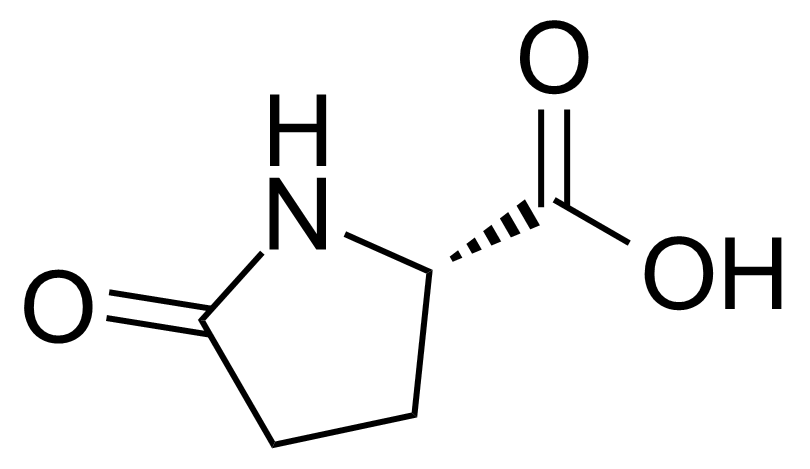
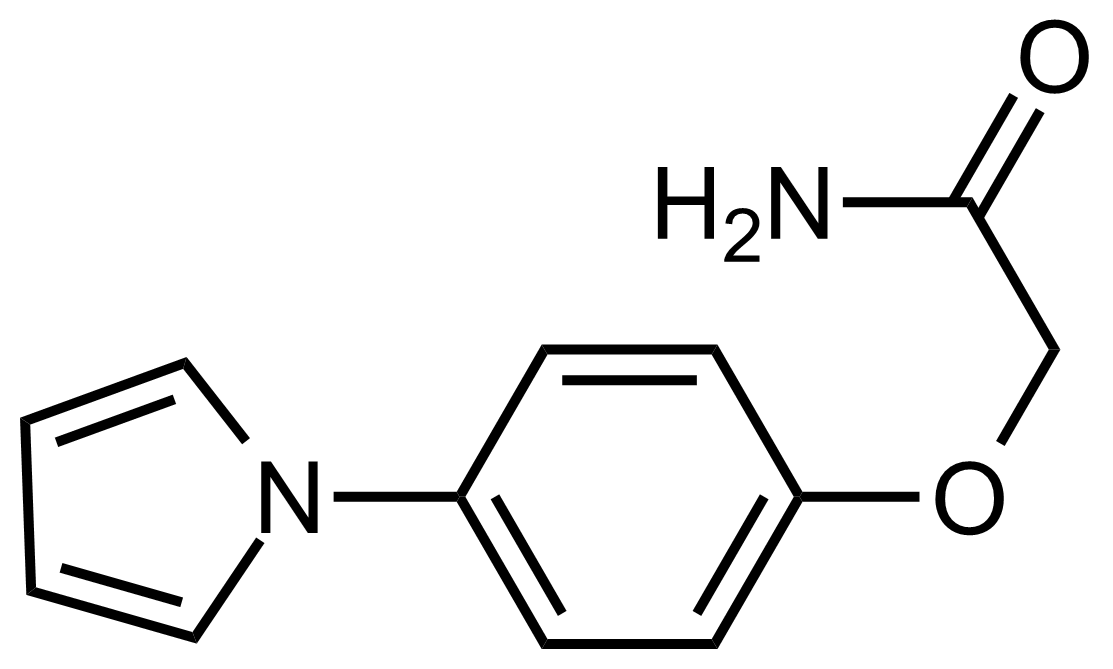
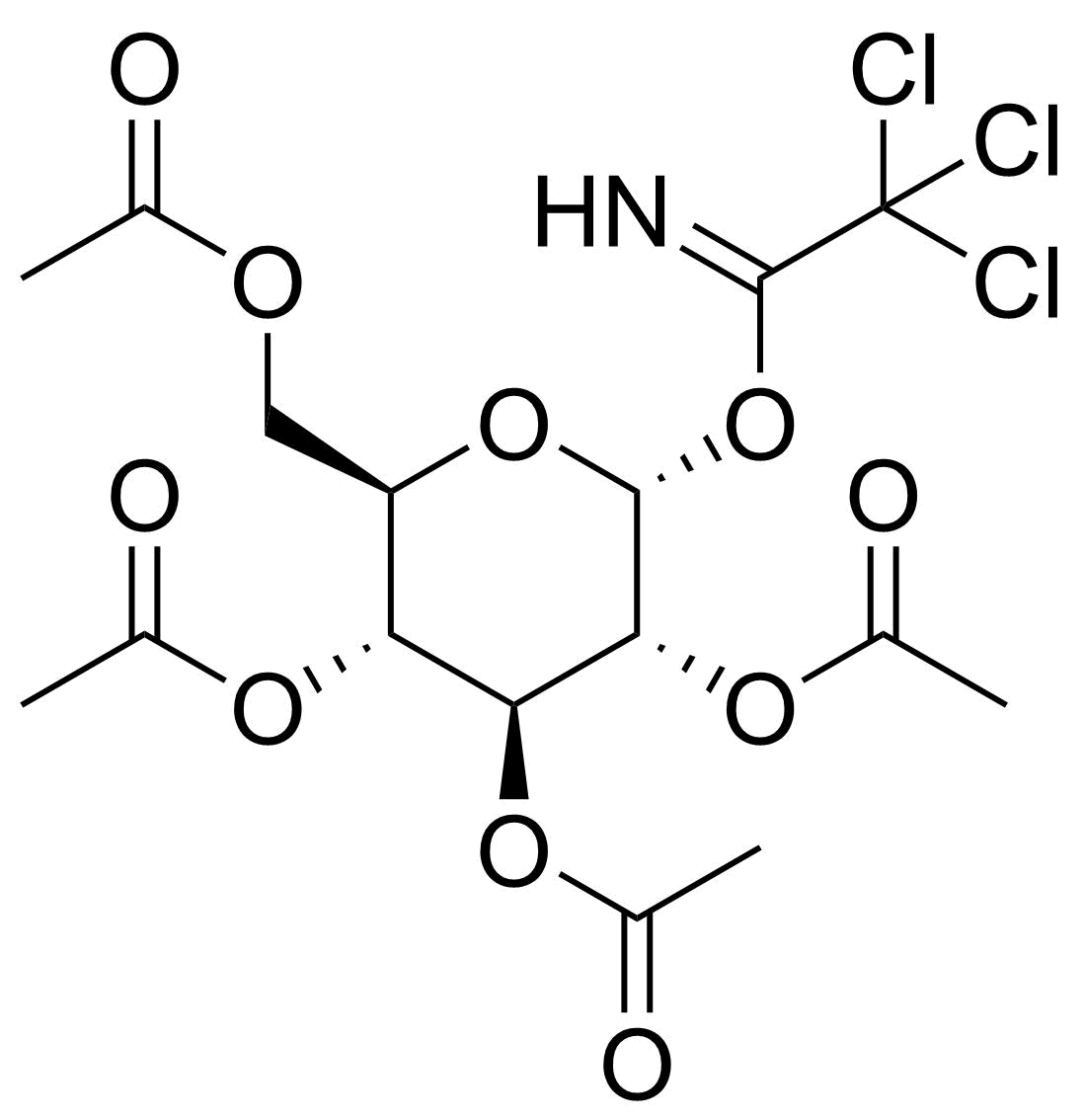
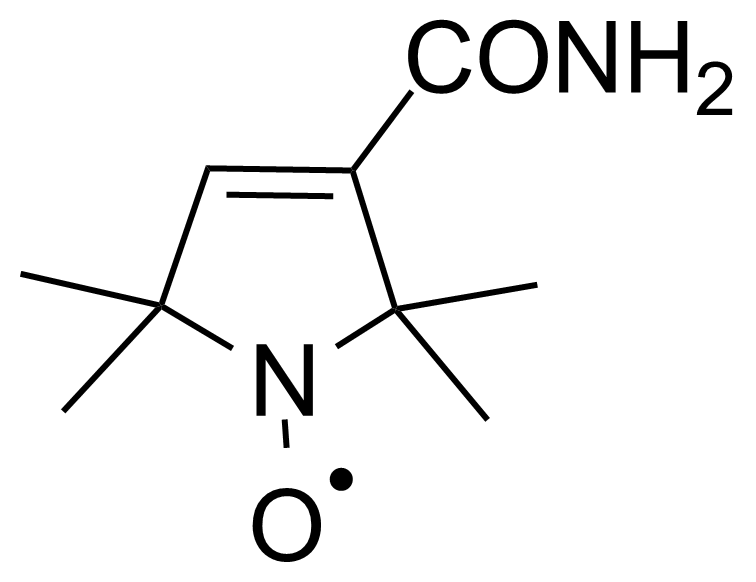
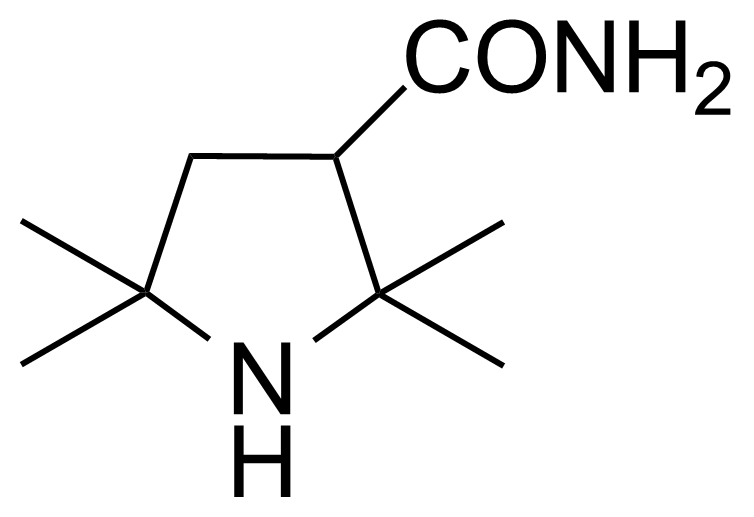
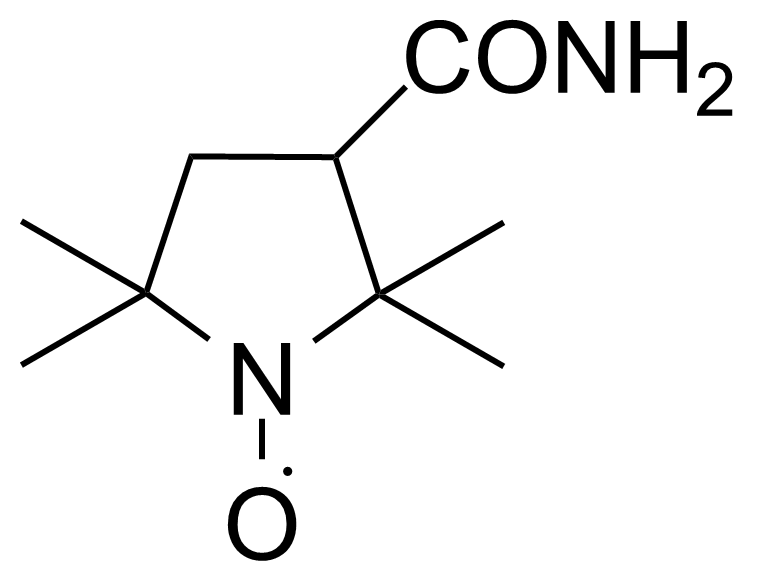
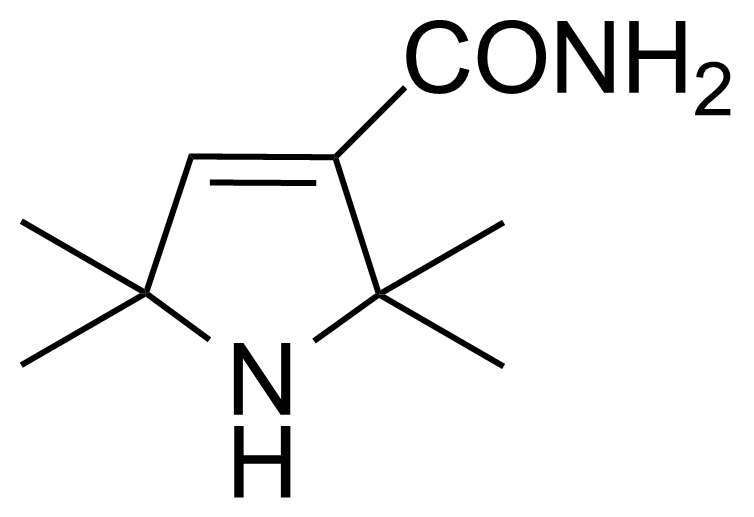
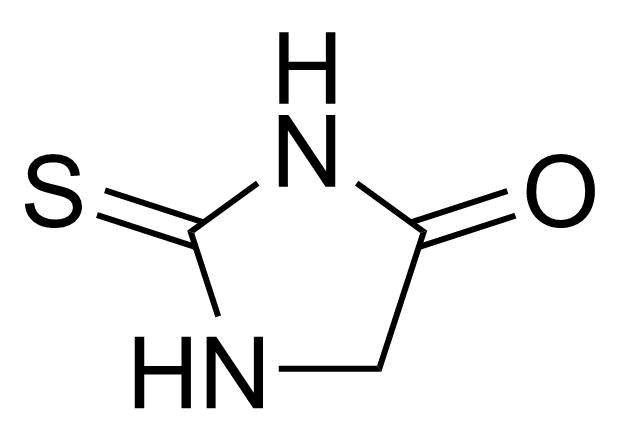
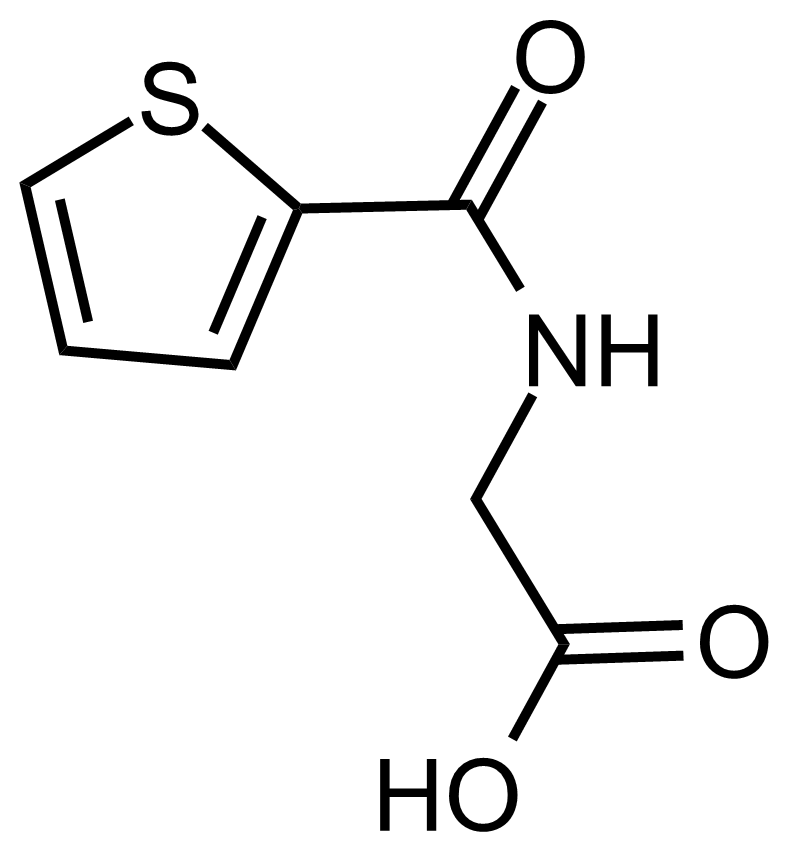
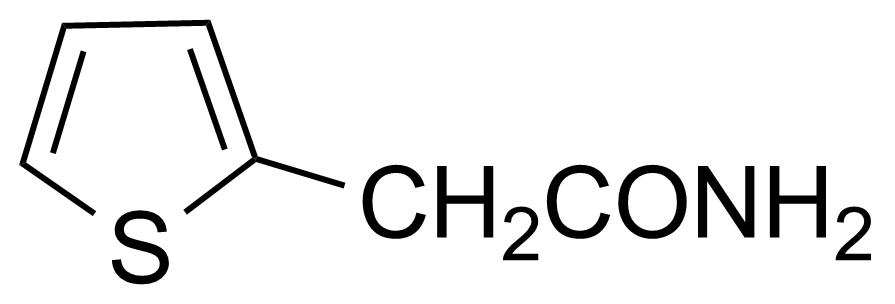
Carboxamides / Thioamides / Imidates
Carboxamides or amides are derivatives of carboxylic acid formed by reaction of appropriate amine with esters, chlorides and anhydrides („activated“ carboxylic acid) or conventional methods in peptide synthesis by use coupling agents (HATU, HOBt, or PyBOP). Amides are pervasive in nature and technology (proteins, plastics, drugs). Thioamides are typically prepared by treating amides with phosphorus sulfides (such as phosphorus pentasulfide). Imidates or carboximidates can be thought of as esters formed between a carboximidic acid and an alcohol. They are also known as imino ethers. Imidates are in general formed by the Pinner reaction which proceeds via the acid catalysed attack of nitriles by alcohols in a form of hydrochloride salts (Pinner salts). Carboximidates are good electrophiles and undergo a range of addition reactions or they can act as protecting group for alcohols.
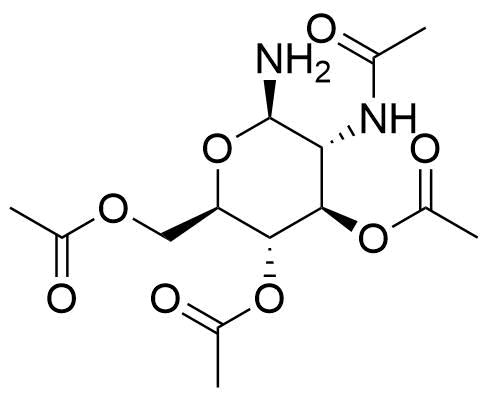
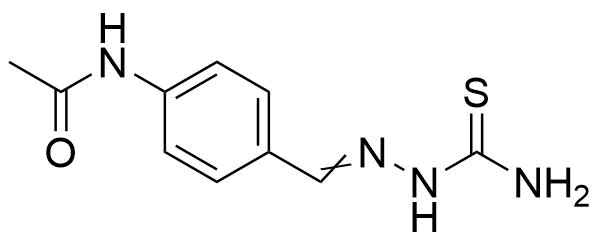
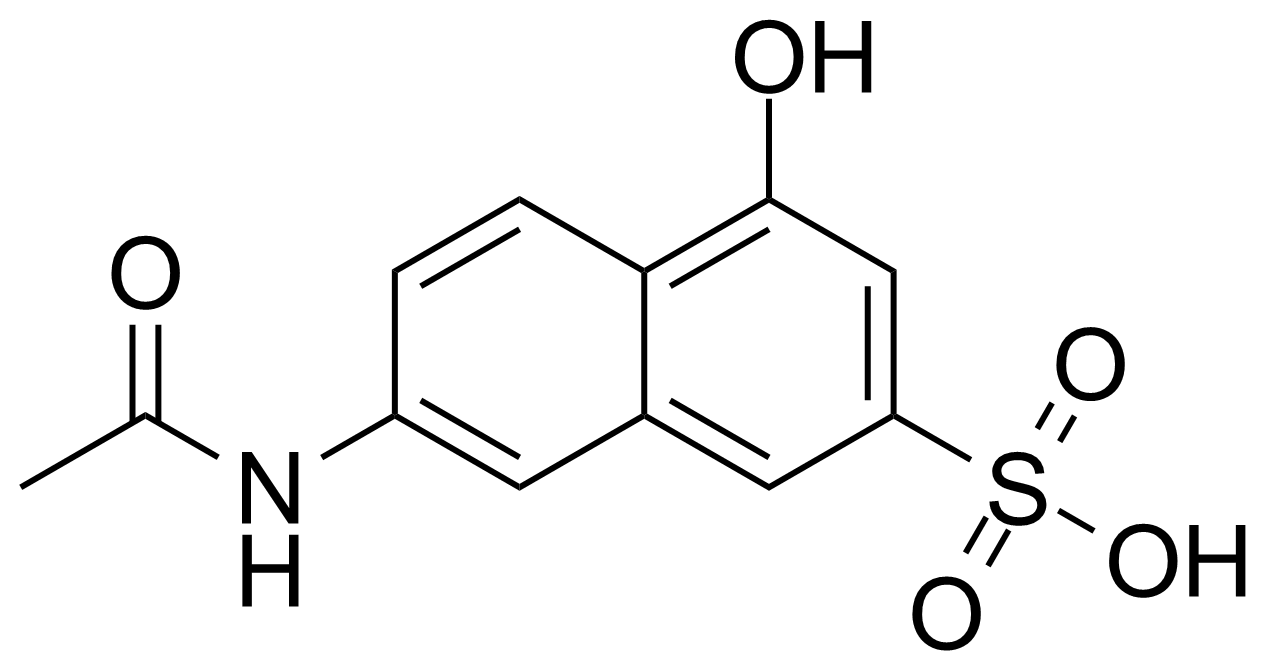
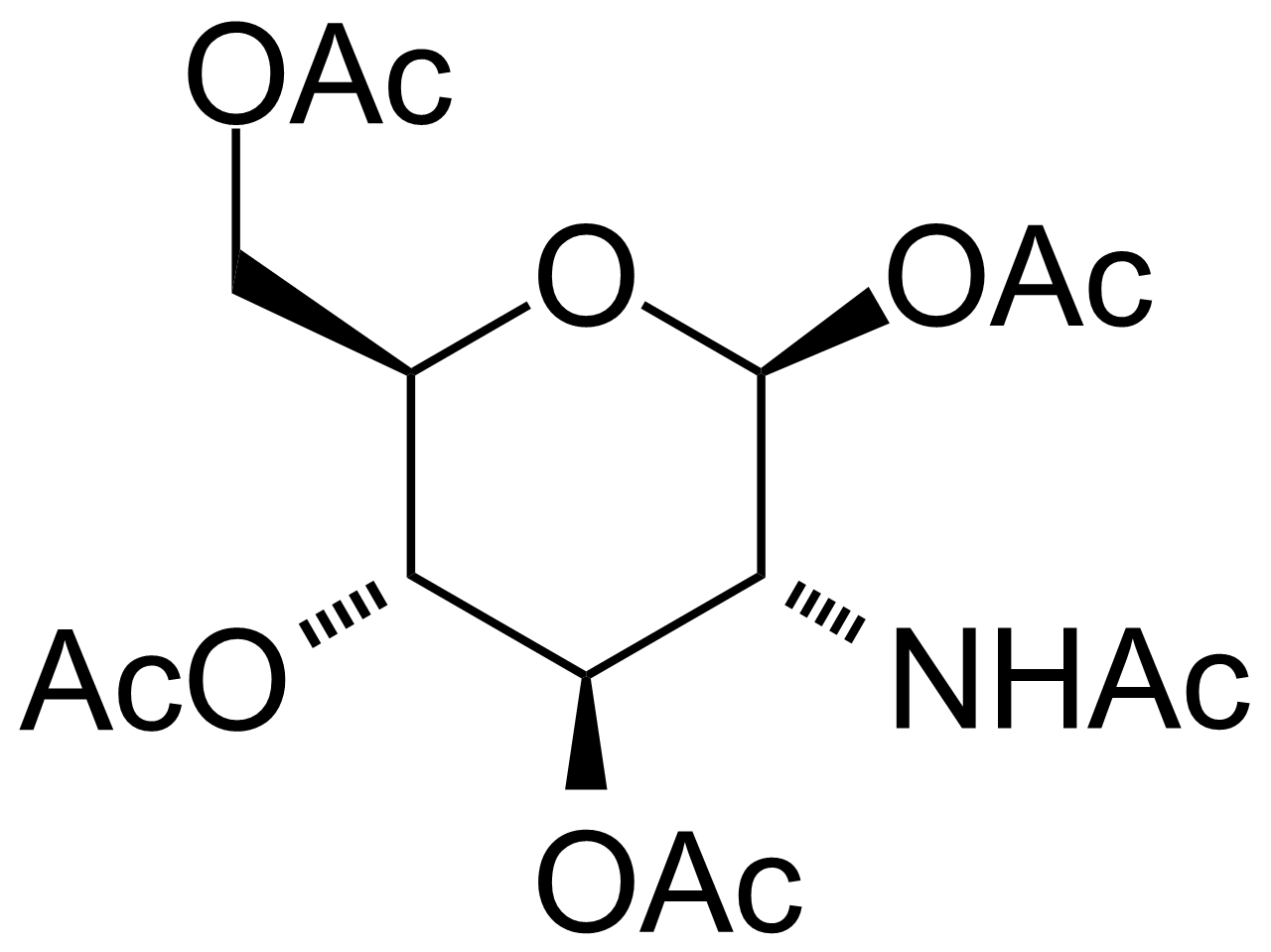
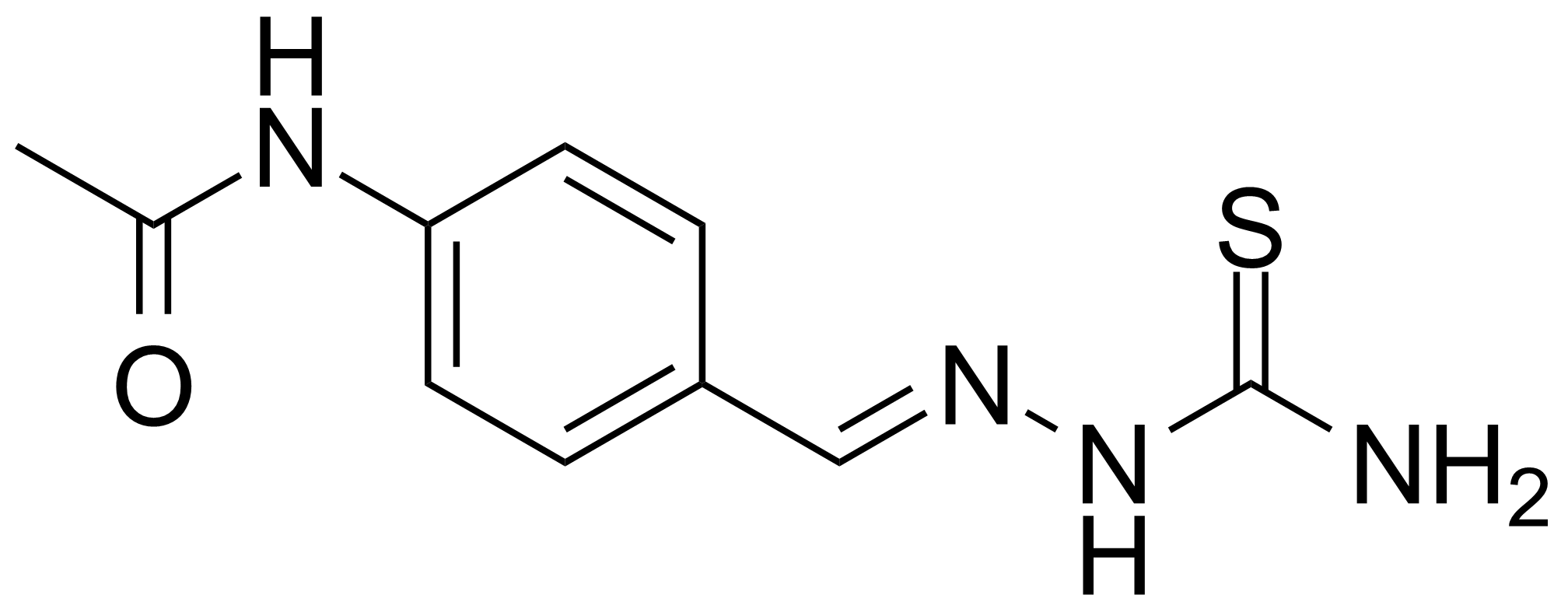
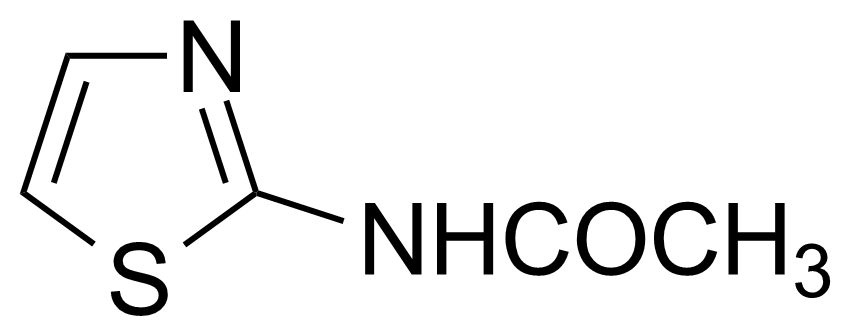

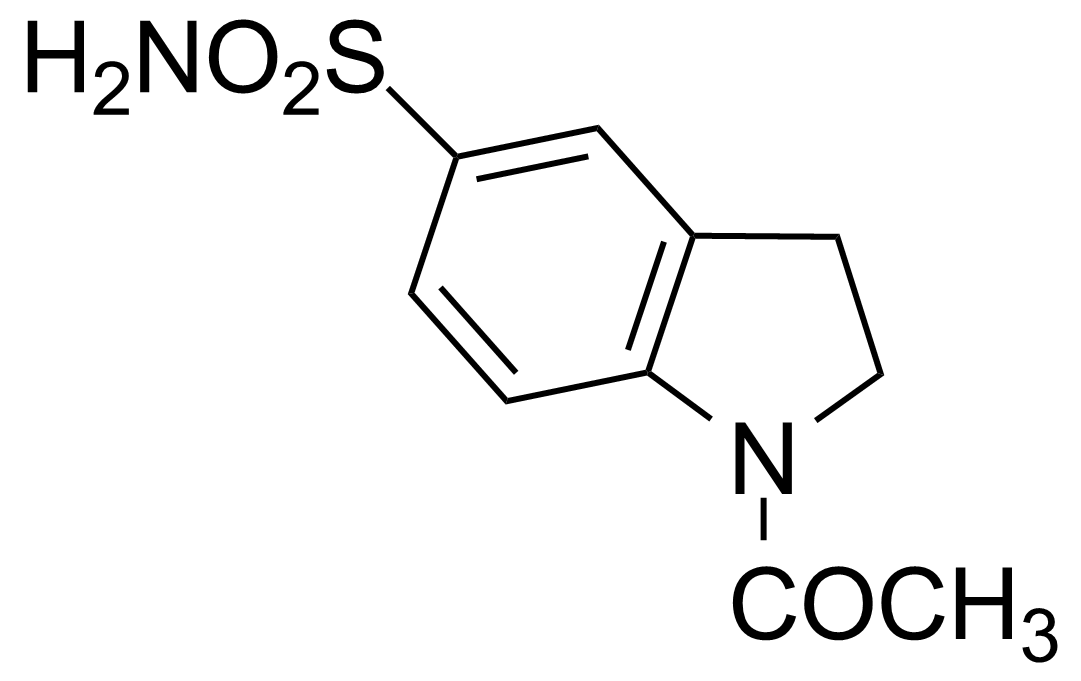
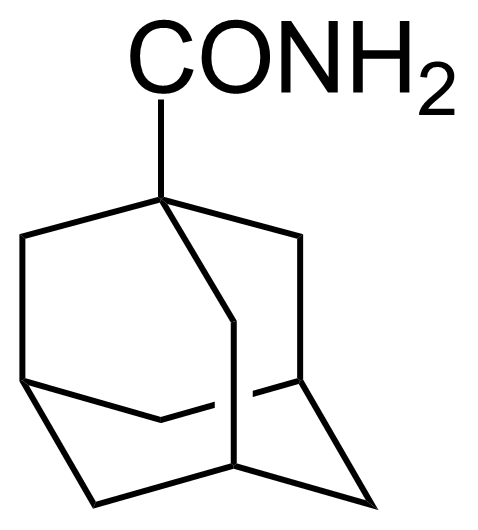
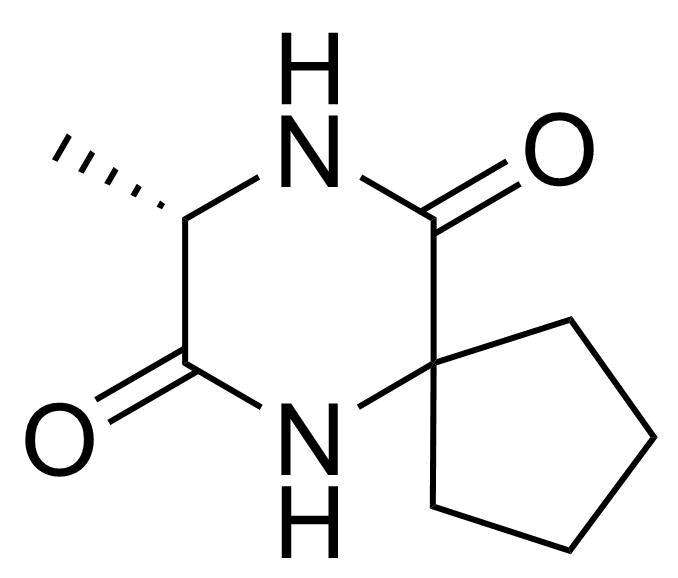
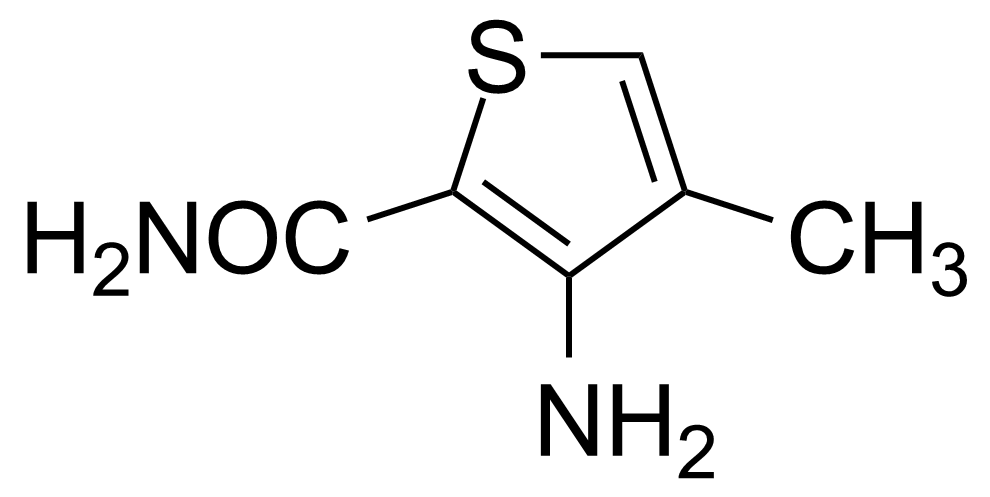
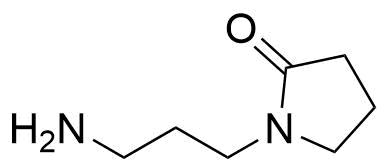
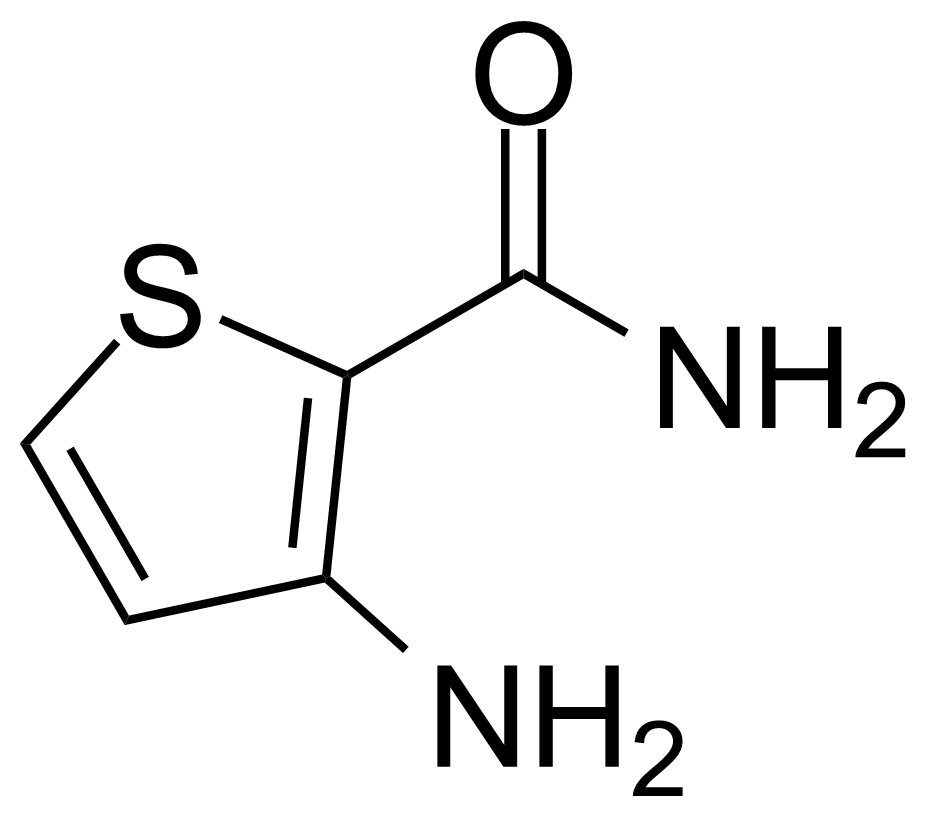
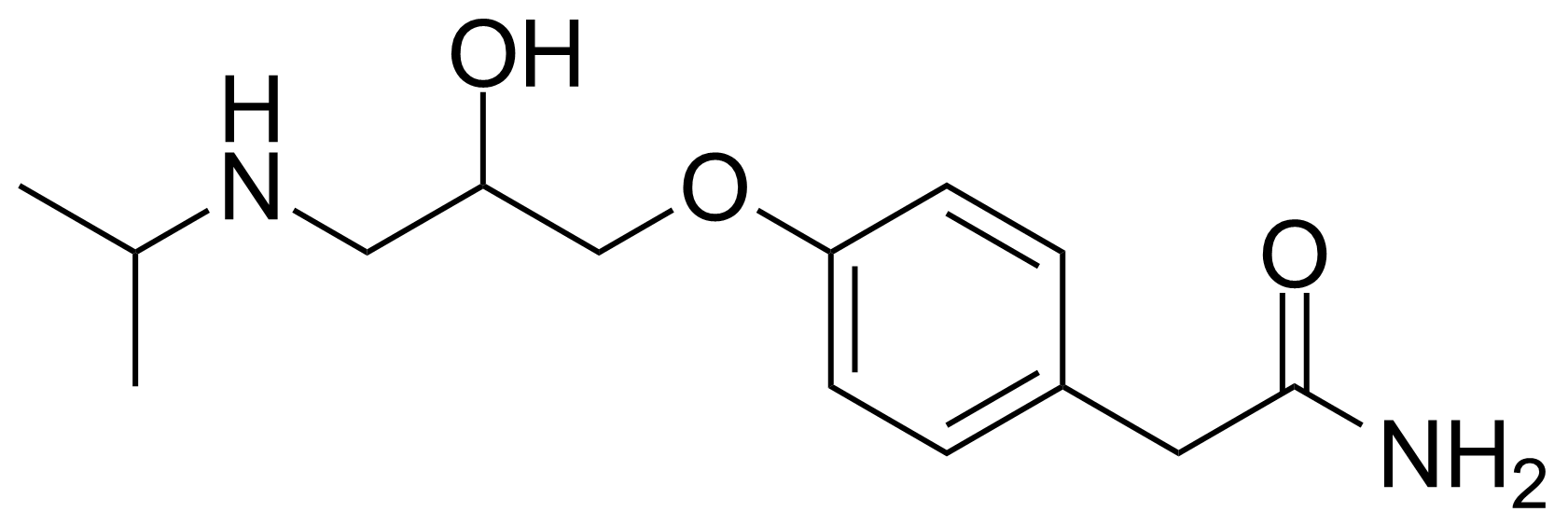
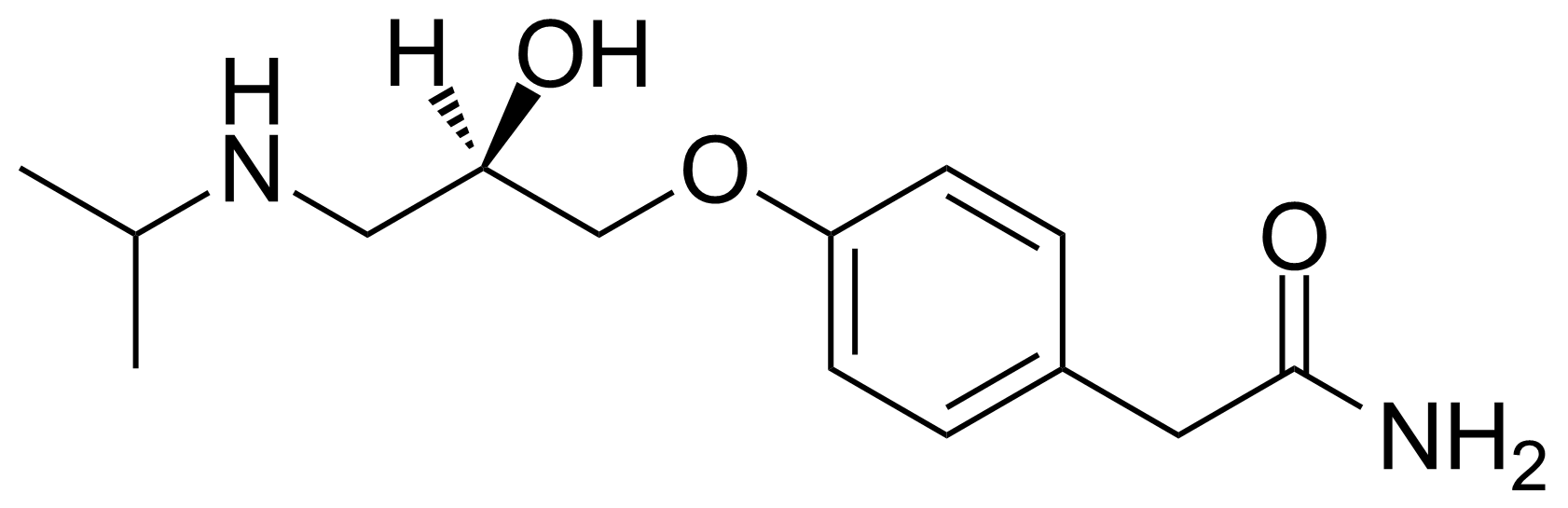
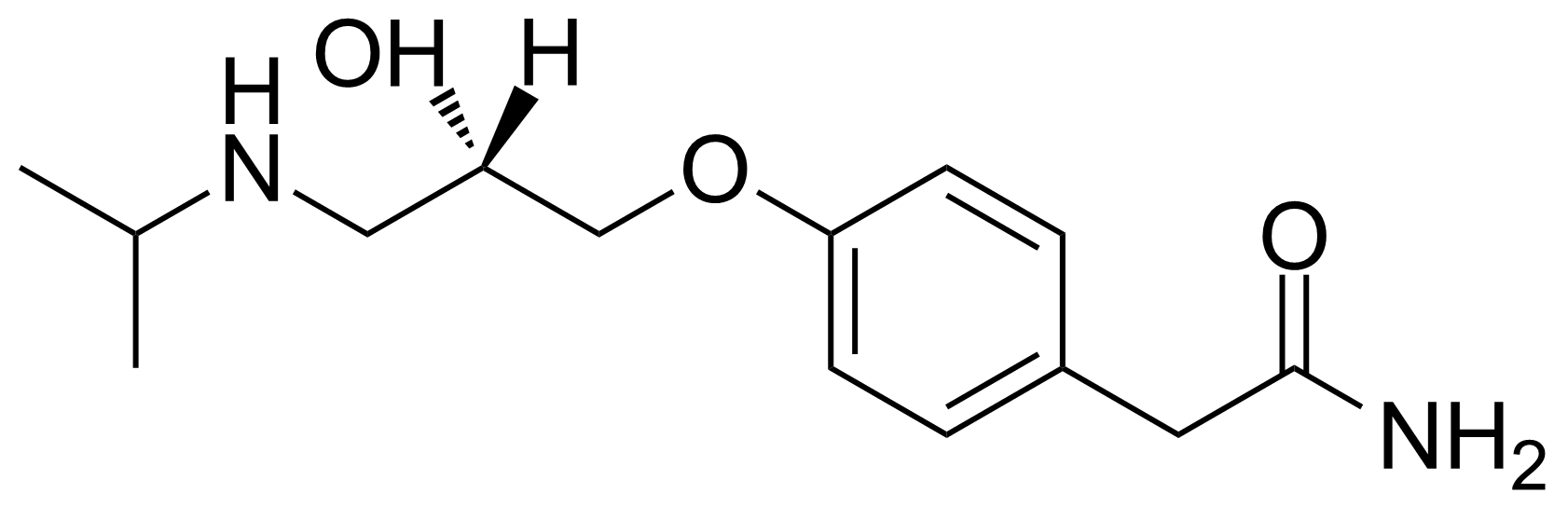
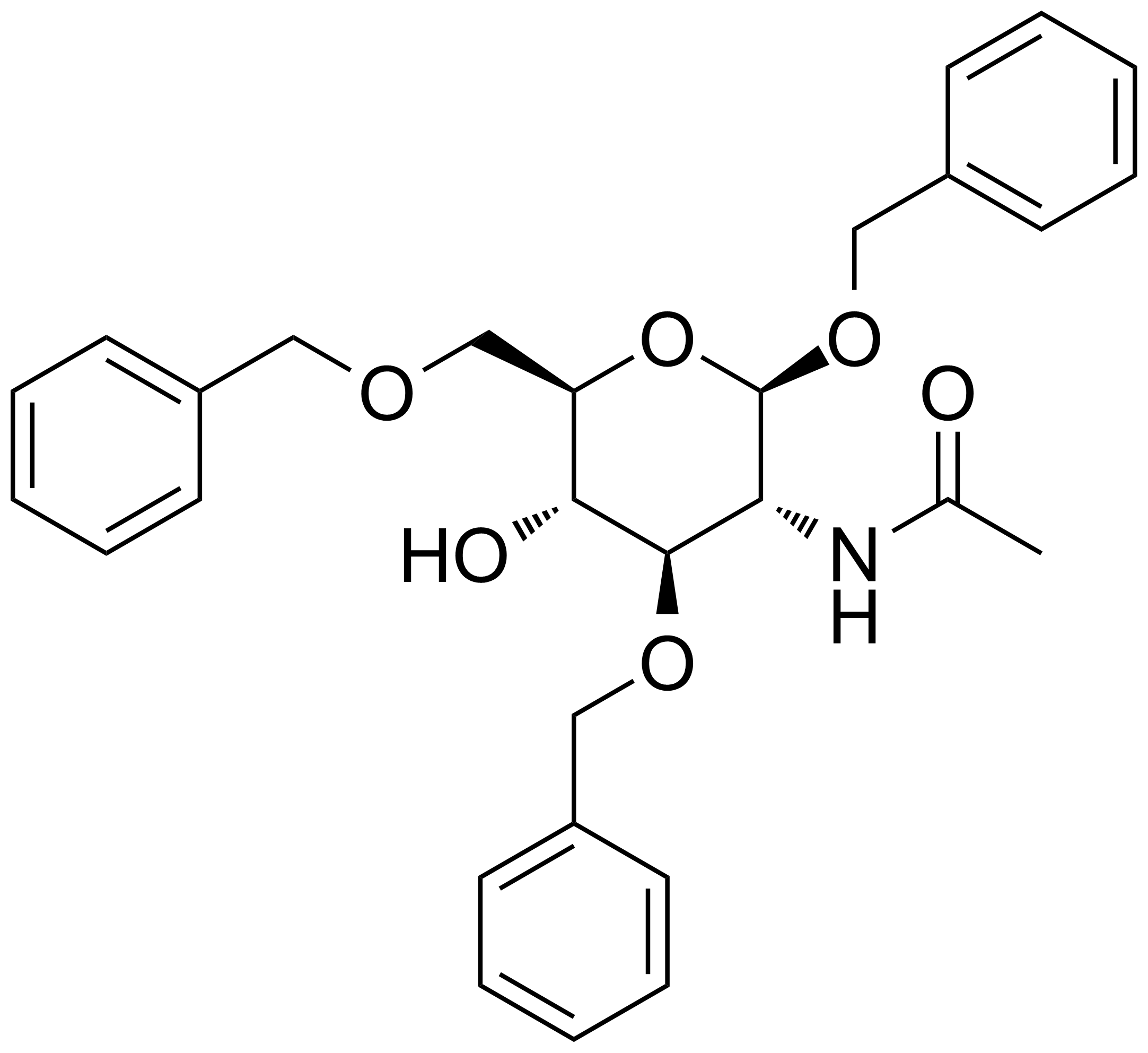
![Structure of 5-Bromobenzo[d]isothiazol-3(2H)-one 1,1-dioxide](https://georganics.sk/wp-content/uploads/2021/05/GEO-02755_5-Bromobenzodisothiazol-32H-one_11-dioxide.png)