 July 04, 2023
July 04, 2023Tetrachlorocyclopropene – description and application
Description of Tetrachlorocyclopropene:
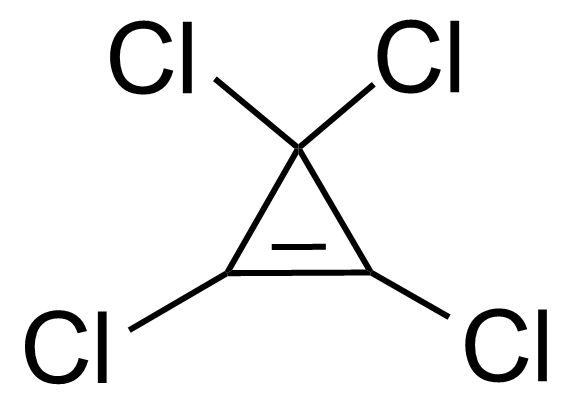
Tetrachlorocyclopropene (TCCP) [6262-42-6] is a colorless liquid with the boiling point of 130–131 °C.[1] It is soluble in common organic solvents and undergoes ring opening in H2O at 25 °C and in alcohols at 50–80 °C. It must be stored at 4 °C in the absence of moisture. Tetrachlorocyclopropene is lachrymator (with the odor of rotten tomatoes) therefore it should be use in a fume hood.[2]
Application of Tetrachlorocyclopropene:
Tetrachlorocyclopropene is a useful precursor for the formation of cyclopropenium salts, cyclopropenylidenes, cyclopropenones, and cyclopropenimines. Treatment of Tetrachlorocyclopropene with aluminum chloride results in a chloride abstraction that generates the trichlorocyclopropenyl ion. This 2? Hückel aromatic cation has been shown to be significantly more stable than the adamantyl or tert-butyl cations.[3] Tetrachlorocyclopropene is widely used as an entry to cyclopropenium cation chemistry via trichlorocyclopropenium salt, which has been the starting point for the synthesis of a broad variety of heterocycles, e.g. benzimidazoles or 1,5-benzodiazepines,[4] 2-azacalicenium salts,[5] or indolizines.[6] It undergoes thermal ring opening to perchlorovinylcarbene which can be efficiently trapped with a large number of alkenes. Formed intermediates have been widely used in natural and nonnatural product synthesis,[7] including ?- and ?-amino acids,[8] and ?-lactams.[9]
Cyclopropenimines made from TCCP are utilized as enantioselective Brönsted base organocatalysts and as superbases. Structurally similar to guanidines, cyclopropenimines possess a higher basicity comparable to the bicyclic guanidines, such as triazabicyclodecene (TBD), and phosphazenes.[10] Other cyclopropenimines have function as pH-responsive fluorescent probes[11] (“Janus” sponge) and as phase-transfer catalysts.[12]
Tetrachlorocyclopropene has been employed as a highly reactive dienophile in various cycloadditions. The Diels–Alder reaction of furans or cyclopentadienes with tetrachlorocyclopropene forms exo-cycloadducts that spontaneously undergo a [2+0] torquoselective sigmatropic rearrangement to give 2,3,4,4-tetrachlorobicyclo[3.2.1]octa2,6-dienes.[13] Tetrachlorocyclopropene provides access to radialenes, cyclic cross-conjugated polyenes with exocyclic double bonds, in two steps. Radialenes are noted for their unusual electronic properties, photophysical properties, and their potential as p-dopants.[14] They are used as doping agents for doping an organic semiconductive matrix material as blocker material, as charge injection layer, as electrode material as well as organic semiconductor, as well as electronic components and organic semiconductive materials using them.[15] Tetrachlorocyclopropene serves as a starting material for the cyclopropenium ions that have raised much attention as organocatalysts and redox active polymers. Furthermore, it was demonstrated that cyclopropenium-based liquid crystals self-assemble into lamellar or columnar mesophases, which are important in polyelectrolytes used for battery materials.[16]
Tetrachlorocyclopropene is used for preparation of cyclopropenium compounds which are capable of forming a columnar liquid crystal mesophase. Liquid crystals are used extensively in a wide range of applications such as displays, solar cells, optoelectronics, molecular sensors and detectors.[17]
Product categorization (Chemical groups):
Main category:
Second level:
_______________________________________________________________________
[2] S. W. Tobey, R. West Tetrahedron. Lett. 1963, 4 (18), 1179. doi:10.1016/S0040-4039(01)90799-3
[3] J.-L. M. Abboud, O. Castaño, M. Herreros, I. Leito, R. Notario, K. Sak J. Org. Chem. 1998, 63 (24), 8995. doi:10.1021/jo981369y
[4] Z. Yoshida, H. Hirai, S. Miki, S. Yoneda Tetrahedron 1989, 45, 3217. doi:10.1016/S0040-4020(01)80147-2
[5] R. Gompper, R. Guggenberger Tetrahedron 1986, 42, 839. doi:10.1016/S0040-4020(01)87490-1
[6] K. A. Smith, K.C. Waterman, A. J. Streitwieser Jr. J. Org. Chem. 1985, 50, 3360. doi:10.1021/jo00218a023
[7] L. Wessjohann, N. Krass, D. Yu, A. de Meijere Chem. Ber. 1992, 125, 867. doi:10.1002/cber.19921250418
[8] M. Es-Sayed, C. Gratkowski, N. Krass, A. I. Meyers, A. de Meijere Synlett 1992, 962. doi:10.1055/s-1992-21545
[9] M. Es-Sayed, T. Heiner, A. de Meijere Synthesis 1993, 57. doi:10.1055/s-1993-22347
[10] J. S. Bandar, T. H. Lambert J. Am. Chem. Soc. 2012, 134 (12), 5552. doi:10.1021/ja3015764
[11] L. Belding, M. Guest, R. Le Sueur, T. Dudding J. Org. Chem. 2018, 83 (12), 6489. doi:10.1021/acs.joc.8b00770
[12] R. Mirabdolbaghi, T. Dudding, T. Stamatatos Org. Lett. 2014, 16 (11), 2790. doi:10.1021/ol501068f
[13] D. C. F. Law, S. W. Tobey J. Am. Chem. Soc. 1968, 90, 2376. doi:10.1021/ja01011a030
[14] Y. Karpov, N. Kiriy, M. Al-Hussein, M. Hambsch, T. Beryozkina, V. Bakulev, S. C. B. Mannsfeld, B. Voit, A. Kiriy Chem. Commun. 2018, 54, 307. doi:10.1039/C7CC08671G
[15] O. Zeika, S. Willmann, S. Dorok, A. Werner, Ch. Bachmann Radialene compounds and their use 2011, NovaLED GmbH US8057712B2.
[16] J. S. Bandar, M. Ebert, R. Forschner, K. Badner, T. H. Lambert, W. Frey, A. Bühlmeyer, M. Brändle, F. Schulz, S. Laschnat Angew. Chem. Int. Ed. 2020, 59 (26), 10557. doi:10.1002/anie.202000824
[17] P. McGonigal Cyclopropenium compounds 2022, University Of Durham WO2022269235A1.