 November 02, 2023
November 02, 2023N-Ethylmaleimide – description and application
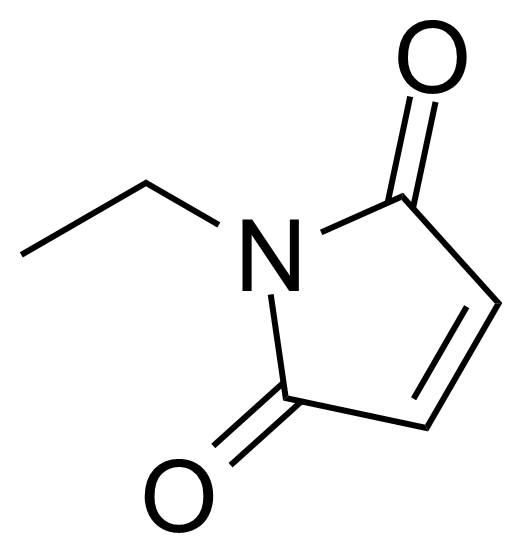
N-Ethylmaleimide (NEM) [128-53-0] is a white crystalline solid with the melting point of 44 °C.[1] It is derived from maleic acid. N-Ethylmaleimide can be easily prepapred from maleic anhydride by reaction with ethylamine in glacial acetic acid under reflux.[2]
Application of N-Ethylmaleimide:
It is a Michael acceptor that is reactive toward nucleophiles such as thiols therefore it is commonly used to modify cysteine residues in proteins and peptides. NEM has been widely used to probe the functional role of thiol groups in enzymology. NEM is an irreversible inhibitor of all cysteine peptidases.[3] N-Ethylmaleimide was used by Arthur Kornberg (Nobel Prize in Physiology or Medicine 1959) and colleagues to knock out DNA polymerase III to compare its activity to that of DNA polymerase I.[4]
It blocks vesicular transport. In lysis buffers, 20 to 25 mM of NEM is used to inhibit de-sumoylation of proteins for Western Blot analysis. NEM inactivates endogenous deubiquitinating enzymes and specifically inhibits phosphate transport in mitochondria.[5]
It was used in synthesis of novel chromone-maleimide hybrids as anti-inflammatory agents for acute lung injury.[6] NEM can be used as a valuable reagent for intramolecular 1,3-dipolar cycloadditions[7], oxidative [3 + 2] cycloadditions with benzylamines[8] or Diels-Alder reactions.[9]
Product categorization (Chemical groups):
Main category:
Second level:
_______________________________________________________________________
[2] R. Mandal, B. Emayavaramban, B. Sundararaju Org. Lett. 2018, 20 (10), 2835. doi:10.1021/acs.orglett.8b00761
[3] J. D. Gregory J. Am. Chem. Soc. 1955, 77 (14), 3922. doi:10.1021/ja01619a073
[4] N. Kresge, R. D. Simoni, R. L. Hill J. Biol. Chem. 2005, 280 (49), e46. doi:10.1016/S0021-9258(20)59088-1
[5] B. Wang, T. Wang, H. Zhu, R. Yan, X. Li, C. Zhang, W. Tao, X. Ke, P. Hao, Y. Qu Cell. Rep. 2022, 38 (12), 110538. doi:10.1016/j.celrep.2022.110538
[6] Y. Zhang, Z. Xu, L. Zhan, Y. Gao, B. Zheng, Y. Zhou, Y. Sheng, G. Liang, Z. Song Bioorg. Chem. 2022, 128, 106049. doi:10.1016/j.bioorg.2022.106049
[7] D. S. Reddy, I. M. Novitskiy, A. G. Kutateladze Angew. Che. Int. Ed. 2022, 61 (4), e202112573. doi:10.1002/anie.202112573
[8] A. Das, N. Chatani Chem. Comm. 2022, 58, 1123. doi:10.1039/D1CC06622F
[9] S. Higson, F. Subrizi, T. D. Sheppard, H. C. Hailes Green. Chem. 2016, 18, 1855. doi:10.1039/C5GC02935J