 February 07, 2022
February 07, 2022(E)-3-(3-Fluorophenyl)acrylic acid – general description and preparation
General description of (E)-3-(3-Fluorophenyl)acrylic acid:
(E)-3-(3-Fluorophenyl)acrylic acid [20595-30-6], 3-fluorocin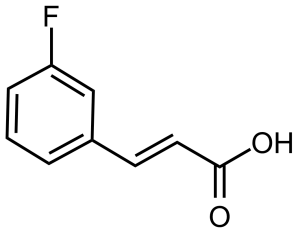
It is known that 3-fluorocinnamic acid can cause serious damage if swallowed and can be irritating in contact with skin or eyes (H301, H315, H319).[2]
Preparation:
3-Fluorocinnamic acid can be prepared by Knowenagel-Doebner reaction of malonic acid or malonic ester with corresponding aldehyde. [3] It is also formed as side product in kinetic resolution of β-phenylalanine derivatives via selective conversion of a single enantiomer to the corresponding acrylic acid. [4] Recent research also showed that it can be prepared by Suzuki coupling of corresponding boronic acid with carbon dioxide. [5]
_______________________________________________________________________
[2] https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/notification-details/29188/647793
[3](a) W. Szymanski, B. Wu, B. Weiner, S. de Wildeman, B. L. Feringa, D. B. Janssen, J. Org. Chem. 2009, 74, 9152–9157; Doi: 10.1021/jo901833y. (b) G. Schieniann, W. Winkelmüller J. prakt. Chem. 1932, 325, 101 – 107. Doi: 10.1002/prac.19321350303. (c) J. Luo, S. L. Castle, R. N. Castle, J. Heterocyclic. Chem. 1990, 27, 2047. Doi: 10.1002/jhet.5570270737. (d) L. Tan, Q. Zhou, W. Yan, J. Sun, A. P. Kozikowski, S. Zhao, X. Huang, J. Cheng, J. Med. Chem. 2020, 63, 4579 – 4602. Doi: 10.1021/acs.jmedchem.9b01835.
[4] (a) A. Varga, P. Csuko, O. Sonesouphap, G. Bánóczi, M. I. Tosa, G. Katona, Z. Molnar, L. C. Bencze, L. Poppe, C. Paizs, Catal. Today 2021, 366, 185 – 194. Doi: 10.1016/j.cattod.2020.04.002. (b) I. Rowles, B. Groenendaal, B. Binay, K. J. Malone, S. C. Willies, N. J. Turner, Tetrahedron, 2016, 46, 7343-7347. Doi: 10.1016/j.tet.2016.06.026. (c) A. Varga, G. Bánóczi, B. Nagy, L. C. Bencze, M. I. Toşa, Á. Gellért, F. D. Irimie, J. Rétey, L. Poppe, C. Paizs, RSC. Adv. 2016, 61, 1 – 9. Doi: 10.1039/c6ra02964g.
[5] J. Hong, O. S. Nayal, F. Mo, Eur. J. Org. Chem. 2020, 19, 2813 – 2818. Doi: 10.1002/ejoc.202000288.