 December 20, 2021
December 20, 2021Cyclobutanecarboxylic acid
General description of Cyclobutanecarboxylic acid
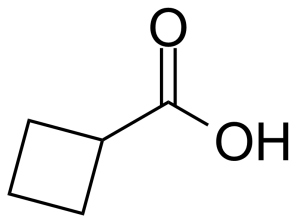
Cyclobutanecarboxylic acid or cyclobutyl formic acid [3721-95-7] is a colorless liquid with the boiling point of 196 °C[1] at atmospheric pressure and melting point around -7 °C[2]. Altought relatively high LD50 (mouse, subcutaneous and intraperitoneal, 1270 mg/kg),[3] this compound is corrosive and can cause severe skin burns and eye damage.
Cyclobutanecarboxylic acid is usually prepared by the hydrolysis and decarboxylation of diethyl cyclobutanedicarboxylate, formed in reaction of trimethylene dibromide with ethyl malonate and sodium in ethanol.[4] Another preparation is based on the oxidative ring contraction of cyclopentanone with hydrogen peroxide catalysed with selenium dioxide.[5]
Application of Cyclobutanecarboxylic acid
Cyclobutanecarboxylic acid is useful principal reagent for the preparation of various monosubstituted cyclobutane derivatives in the synthesis of biological active compounds and approved drugs as Butorphanol[6], Nalbuphine[7], Boceprevir[8] and others.[9]
Notes
Stable under recommended storage conditions. Conditions to avoid: Heat, flames and sparks. Incompatible materials: Strong oxidizing agents, Strong bases
Product categorization (Chemical groups)
Main category:
Second level:
______________________________________________________________________________________
[2] J. Boeseken, A. G. Lutgerhorst Recl. Trav. Chim. Pays-Bas 1932, 75, 1846.
[3] A. E. Senior, H. S. Sherratt, J. Pharm. Pharmac. 1969, 85.
[4]
Org. Synth. 1943, 23, 16.
Cason, Ch. F. Allen J. Org. Chem. 1949, 14, 1036.
[5] G. B. Payne, C. W. Smith J. Org. Chem. 1957, 22, 1680.
[6] I. Monković, H. Wong, A. W. Pircio, Y. G. Perron, I. J. Pachter, B. Belleau Can. J. Chem. 1975, 53, 3094.
[7] R.S. Vardanyan, V.J. Hruby Synthesis of Essential Drugs 2006, Elsevier.
[8] D. S. Bhalerao, A. K. R. Arkala, Y. V. Madhavi, M. Nagaraju, S. R. Gade, U K. S. Kumar, R. Bandichhor, V. H. Dahanukar Org. Process Res. Dev. 2015, 19, 1559.
[9] A. Sergeiko, V. V. Poroikov, L. O. Hanuš, V. M. Dembitsky Open Med. Chem. J. 2008, 2, 26.</div<