 March 13, 2023
March 13, 2023Levulinic acid (LA) – preparation and application
General description and preparation:
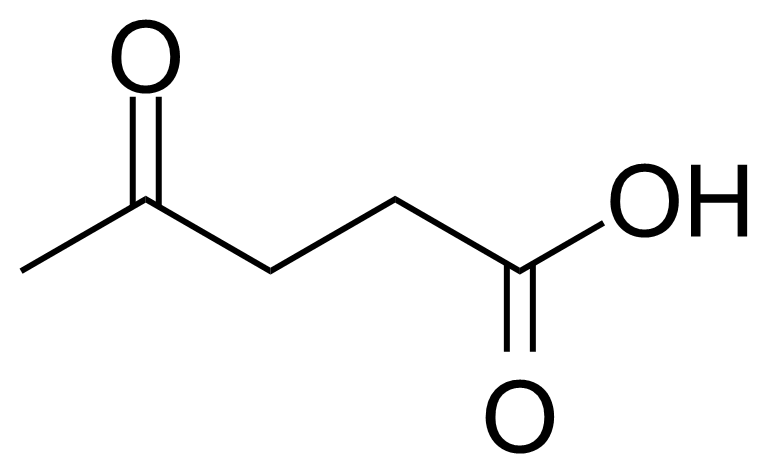
Levulinic acid, or 4-oxo-pentanoic acid, or b-acetylpropionic acid, or g-ketovaleric acid [123-76-2] is an organic acid belonging to keto acids group. In its pure form, it is a white crystalline solid with melting point of 33 °C.[1] It is soluble in water and polar organic solvents. Despite its low toxicity (LD50 1850 mg/Kg, oral, rat), as other acids, it can cause the acid burns and high concentrated solutions are irritating to the skin and mucous membranes.[2] The name, levulinic acid (originally levulinsäure, in german) was suggested by A. v. Grote and B. Tollens in 1874 as it was prepared from L-sugar (L-fructose) known as levulose.[3]
Levulinic acid can be produced by acid hydrolysis of 5-hydroxymethylfufural (5-HMF), or by transformation of biomass/cellulose via formation of monomeric sugar derivatives.[4] In 1953 Quaker Oats developed a continuous process to produce levulinic acid from carbohydrate material such as starch, cellulose, or sugars.[5]
Application of Levulinic acid:
It can be used as a raw material in organic synthesis, especially in the production process of some pharmaceuticals, in the preparation of 5-methyl-2-pyrrolidone,[6] g-valerolactone[7] and angelica lactone.[8] It has been recently found to be useful in the production of plasticizers and its esters as fragrance ingredients (fraistone) are used in the cosmetics production.[9] LA was identified as one of the twelve promising bio-based building blocks that can be subsequently converted to a number of high-value chemicals, fuels or materials.[10]
Product categorization (Chemical groups):
Main category:
Second level:
_______________________________________________________________________
[2] V. Sunjic, J. Horvat, B. Klaic, S. Horvat, Kem. Ind. 1984, 33, 593.
[3] A. V. Grote, B. Tollens, Justus Liebigs Ann. Chem. 1875, 175, 181.
[4] B. F. McKenzie, Org. Synth. 1929, 9, 50. doi:10.15227/orgsyn.009.0050
[5] A. P. Dunlop, P. A. Wells Process for producing levulinic acid 1953, Quaker Oats Co, US2813900
[6] Y. Liu, Y. Wang, Y. Cheng, Z. Wei ChemistrySelect 2022, 7 (26), e202201191. doi:10.1002/slct.202201191
[7] S. K. Hussain, V. K. Velisoju, N. P. Rajan, B. P. Kumar, K. V. R. Chary ChemistrySelect 2018, 3 (22), 6186. doi:10.1002/slct.201800536
[8] C. G. S. Lima, J. L. Monteiro, T. M. Lima, M. W. Paixăo, A. G. Corrĕa ChemSusChem 2017, 11 (1), 25. doi:10.1002/cssc.201701469
[9] C. Antonetti, D. Licursi, S. Fulignati, G. Valentini, A. M. R. Galleti Catalysts 2016, 6 (12), 196. doi:10.3390/catal6120196
[10] S. H. Pyo, S. J. Glaser, N. Rehnberg, R. H. Kaul ACS Omega 2020, 5, 24, 14275. doi:10.1021/acsomega.9b04406