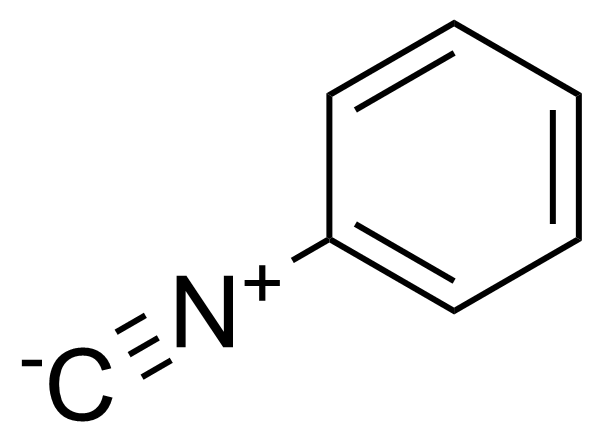
Phenyl isocyanide
Isocyanobenzene
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information

H301 – Toxic if swallowed
H311 – Toxic in contact with skin
H315 – Causes skin irritation
H319 – Causes serious eye irritation
P260 – Do not breathe dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P301+310 – IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P302+352 – IF ON SKIN: Wash with soap and water
P304+340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Phenyl isocyanide is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Phenyl isocyanide in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Phenyl isocyanide was used in a one-pot, six-component, tandem cyclocondensation…
Show full descriptionGeneral description and preparation:
Phenyl isocyanide (PI) or isocyanobenzene [931-54-4] is a colourless liquid with a pungent odour and the boiling point of 61-62 °C/21 mmHg).[1] It is a highly toxic chemical (inhalation/rat LC50 22 mg/m3/4H, oral/mouse LD50 196 mg/kg), asphyxiant and lachrymator.[2] It is moisture sensitive and corrosive. Isonitriles in general were discovered in 1867 by Gautier in the reaction between an alkyl iodide and silver cyanide[3] and Hofmann in the reaction between a primary amine, chloroform, and alcoholic potassium hydroxide.[4] Traditionally, phenyl isocyanide is commercially prepared by dehydration of N-monosubstituted formamides using phosphorus oxychloride in the presence of tertiary amines.[5],[6] Passerini reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence.[7] It is a three component conversion of carbonyl compounds, carboxylic acids and isocyanides into α-acyloxy amides. Its synthetic scope has been increased by employing bifunctional substrated, which are able to undergo secondary reactions.[8]Application of Phenyl isocyanide:
The popularity of the isocyanides began really in 1959 with the introduction of the four component reaction of isocyanides, later named after its inventor as Ugi reaction.[9] Aducts of this reaction are amines (ammonia, mono– and disubstituted amines, hydroxylamine, hydrazine and its suitable derivatives), carbonyl compounds, acid components and related compounds (water, thiosulfates, hydrogen selenide, hydrazoic acid, hydrogen cyanate and thiocyanate, aminocyanic acid, carboxylic acids and thioacids, alkoxycarboxylic acids) and isocyanides.[10] Since then many syntheses of unusual molecules were introduces by new types of isocyanide multicompotent reactions.[11] Recently, phenyl isocyanide was used in a one-pot, six-component, tandem cyclocondensation/Ugi/click reaction sequence, which led to highly complex biologically significant quinoxaline-pseudopeptide-triazole pharmacophores.[12]Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| Benzyl isocyanide | 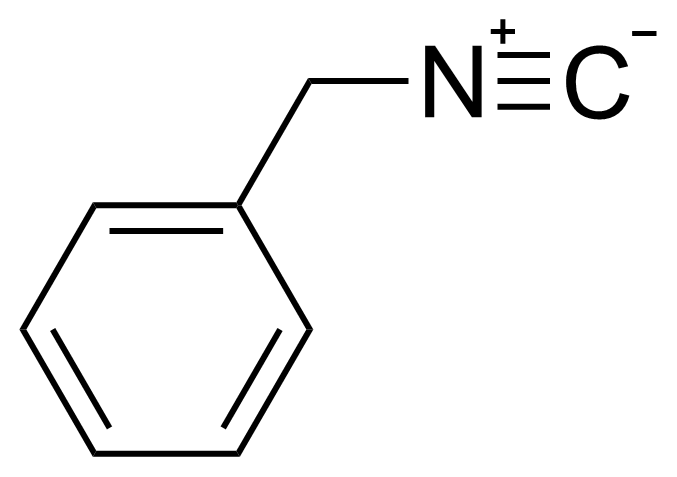 | [10340-91-7] | GEO-02805 | |
| 4-Chlorophenyl isocyanide | 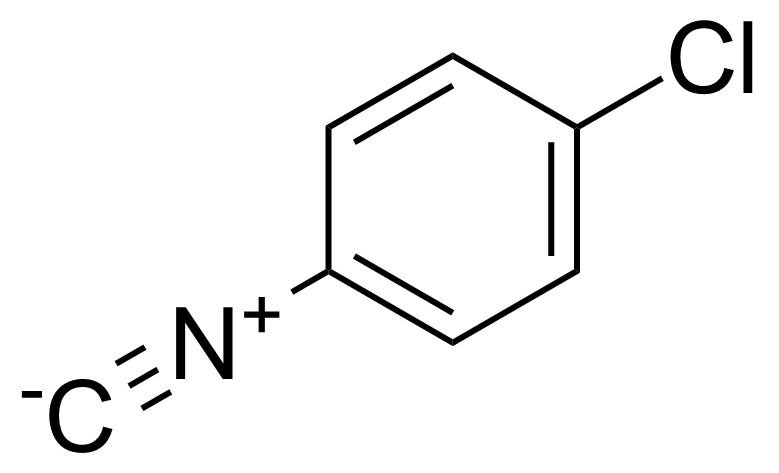 | [1885-81-0] | GEO-03705 | |
| 3-Chlorophenyl isocyanide | 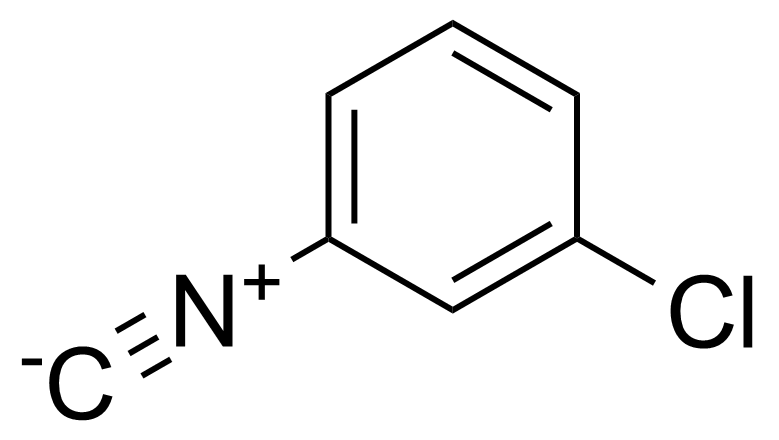 | [32686-54-7] | GEO-03704 | |
| Cyclohexyl isocyanide | 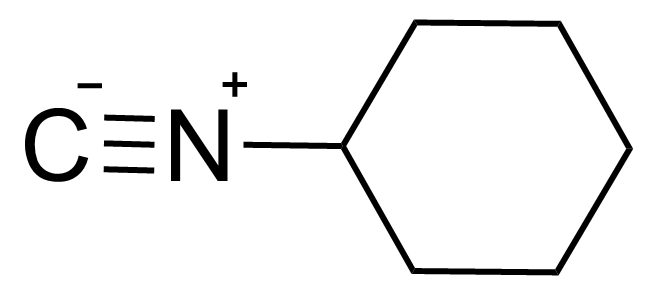 | [931-53-3] | GEO-00871 | |
| Cyclopentylisocyanide | 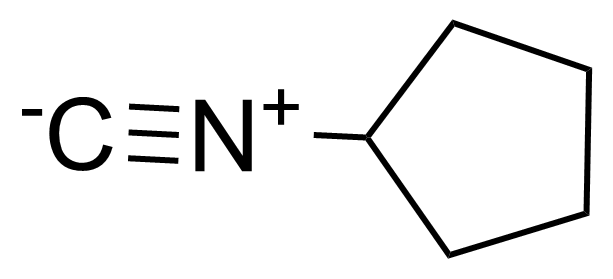 | [68498-54-4] | GEO-02903 | |
| 3,5-Difluoro-1-(isocyanomethyl)benzene | 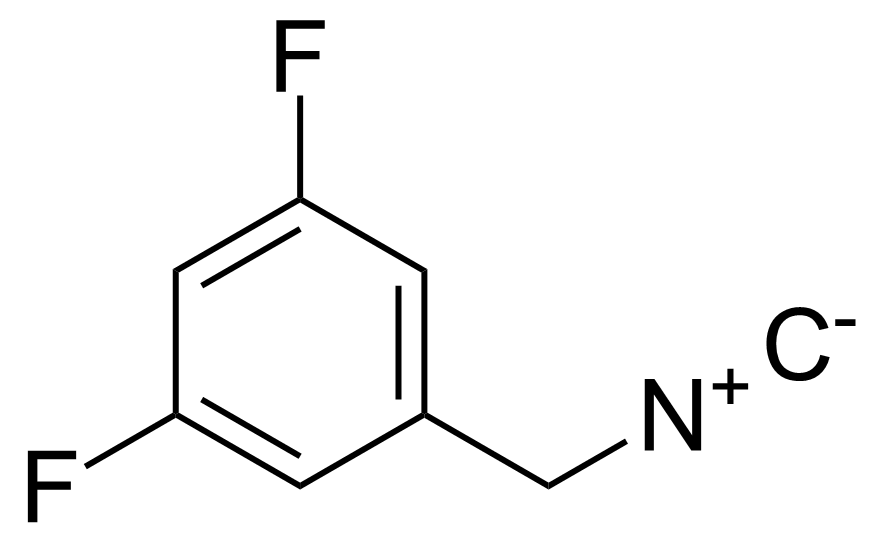 | N/A | GEO-03696 | |
| 2,4-Difluoro-1-(isocyanomethyl)benzene | 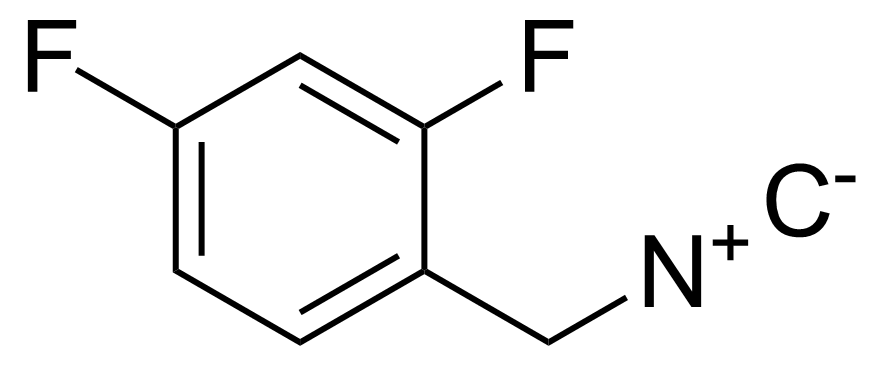 | [730964-55-3] | GEO-03698 | |
| (S)-(-)-alpha-Methylbenzyl isocyanide | 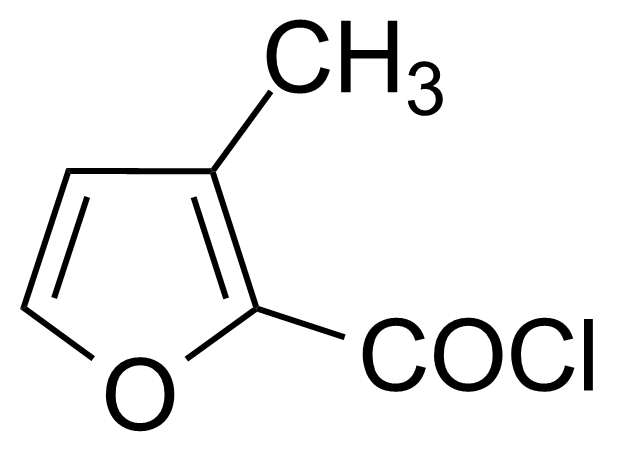 | [21872-32-2] | GEO-03775 | |
| a-Methylbenzyl isocyanide | 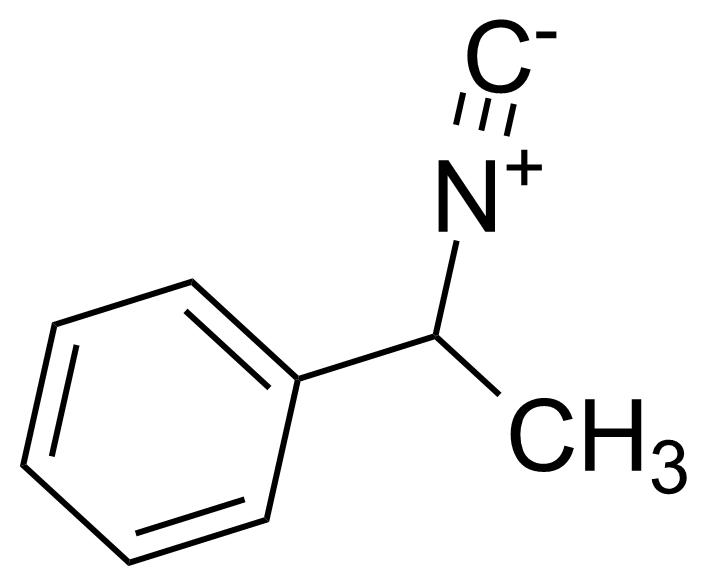 | [17329-20-3] | GEO-01780 | |
| Methyl 2-isocyanoacetate | 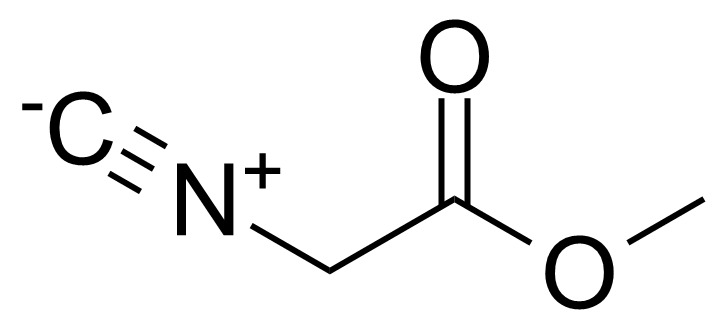 | [39687-95-1] | GEO-03019 |