Nouveau
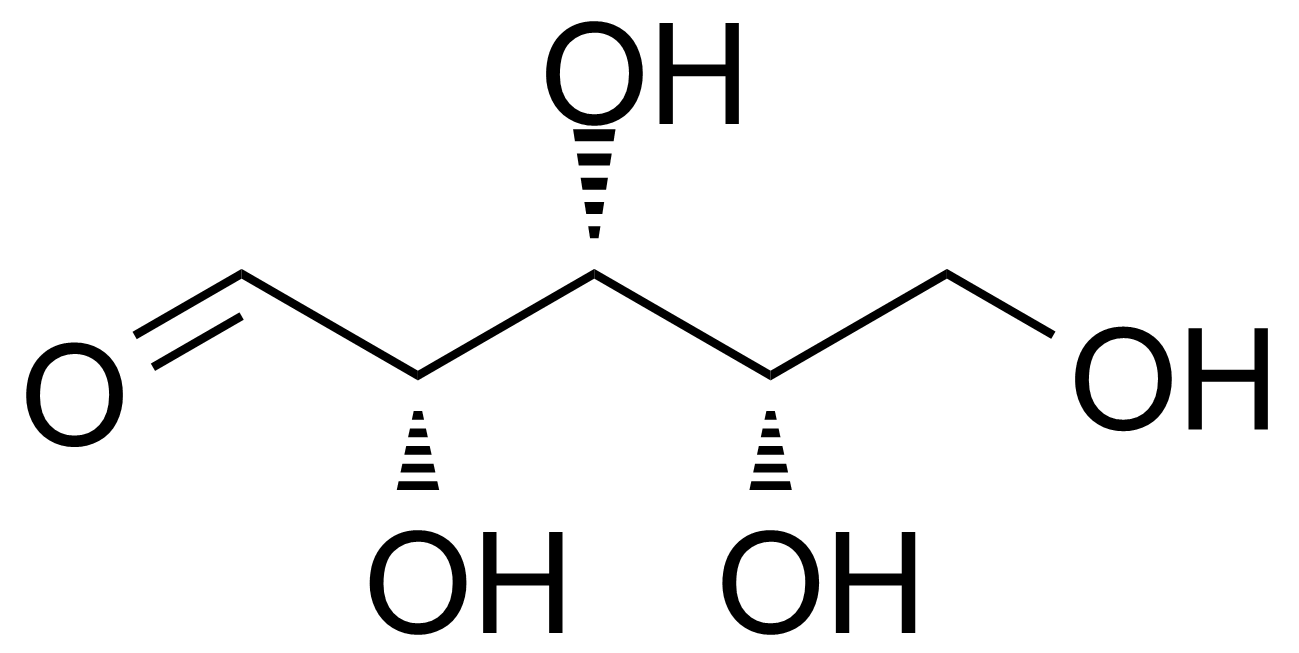
L-Xylose
Numéro CAS[609-06-3]
G-codeGEO-02456
Numéro CE210-174-1
Formule moléculaireC5H10O5
Poids moléculaire150,13
Synonymes
L-(-)-xylose ; (2S,3S,4S)-oxane-2,3,4,5-tetrol ; L-Xylopyranose
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires
Ce produit n’a pas été classé.
Catégorisation des produits
Special offer :
Catégorie principale
Deuxième niveau
Description
L-Xylose est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des L-Xylose de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
L-Xylose [609-06-3] is a rare monosaccharide of the aldopentose type first isolated from wood, and named for it (ancient greek: ξύλον, xylon, "wood"). It is a white crystalline solid with the melting point of 144-145 °C.[1] L-Xylose can be prepared by chemical route from D-gluconolactone [2] or from D-sorbitol.[3],[4] Enzymatic isomerization of the ketosugar L-xylulose to L-xylose has been presented as an alternative for low yields chemical synthesis. The starting material, L-xylulose can be produced by oxidation of the relatively cheap polyol, xylitol, using natural bacterial isolates as whole cell catalysts.[5]
L-Xylose [609-06-3] is a rare monosaccharide of the aldopentose type first isolated from wood, and named for it (ancient greek: ξύλον, xylon, "wood"). It is a white crystalline solid with the melting point of 144-145 °C.[1] L-Xylose can be prepared by chemical route from D-gluconolactone [2] or from D-sorbitol.[3],[4] Enzymatic isomerization of the ketosugar L-xylulose to L-xylose has been presented as an alternative for low yields chemical synthesis. The starting material, L-xylulose can be produced by oxidation of the relatively cheap polyol, xylitol, using natural bacterial isolates as whole cell catalysts.[5]
Application of Furoin:
L-xylose is used in organic synthesis as a chiral building block. It was used for the synthesis of L-ascorbic acid.[6] Novel L-xylose derivatives have recently been reported to act as inhibitors of urinary glucose reabsorption, which suggests that they may find use in diabetes treatment.[7] Polyhydroxypyrrolidines derived from l-xylose have shown antitumor and anti-HIV properties and act as potent α- and β-glucosidase inhibitors, which also is of relevance for the development of diabetes drugs.[8]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________[1] T. G. Bonner, E. J. Bourne, S. E. Harwood, D. Lewis J. Chem. Soc. 1965, 121. doi:10.1039/JR9650000121 [2] W. B. Yang, S. S. Patil, C. H. Tsai, C. H. Lin, J. M. Fang Tetrahedron 2002, 58 (2), 253. doi:10.1016/S0040-4020(01)01146-2 [3] E. Dimant, M. Banay J. Org. Chem. 1960, 25 (3), 475. doi:10.1021/jo01073a621 [4] R. C. Hockett Production of l-xylose 1952, HEINZ M WUEST, US2584129A. [5] A. Usvalampi, O. Turunen, J. Valjakka, O. Pastinen, M. Leisola, A. Nyyssölä Enzyme. Microb. Technol. 2012, 50 (1), 71. doi:10.1016/j.enzmictec.2011.09.009 [6] L. L. Salomon, J. J. Burns, C. G. King J. Am. Chem. Soc. 1952, 74 (20), 5161. doi:10.1021/ja01140a051 [7] N. C. Goodwin, R. Mabon, B. A. Harrison, M. K. Shadoan, Z. Y. Almstead, Y. Xie, J. Healy, L. M. Buhring, C. M. DaCosta, J. Bardenhagen, F. Mseeh, Q. Liu, A. Nouraldeen, A. G. E. Wilson, S. D. Kimball, D. R. Powell, D. B. Rawlins J. Med. Chem. 2009, 52 (20), 6201. doi:10.1021/jm900951n [8] J. B. Behr, G. Guillerm Tetrahedron Lett. 2007, 48 (13), 2369. doi:10.1016/j.tetlet.2007.01.125
Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
| Nouveau | 1-Acenaphthenol |  | [6306-07-6] | GEO-00001 |
| Nouveau | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid |  | [6334-97-0] | GEO-04013 |
| Nouveau | 1-Adamantanemethanol |  | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose |  | [7283-09-2] | GEO-04660 | |
| Nouveau | D-Allose |  | [2595-97-3] | GEO-00057 |
| L-Allose |  | [39392-62-6] | GEO-04661 | |
| Nouveau | 2-(Allyloxy)phenol |  | [1126-20-1] | GEO-04471 |
| D-Altrose | 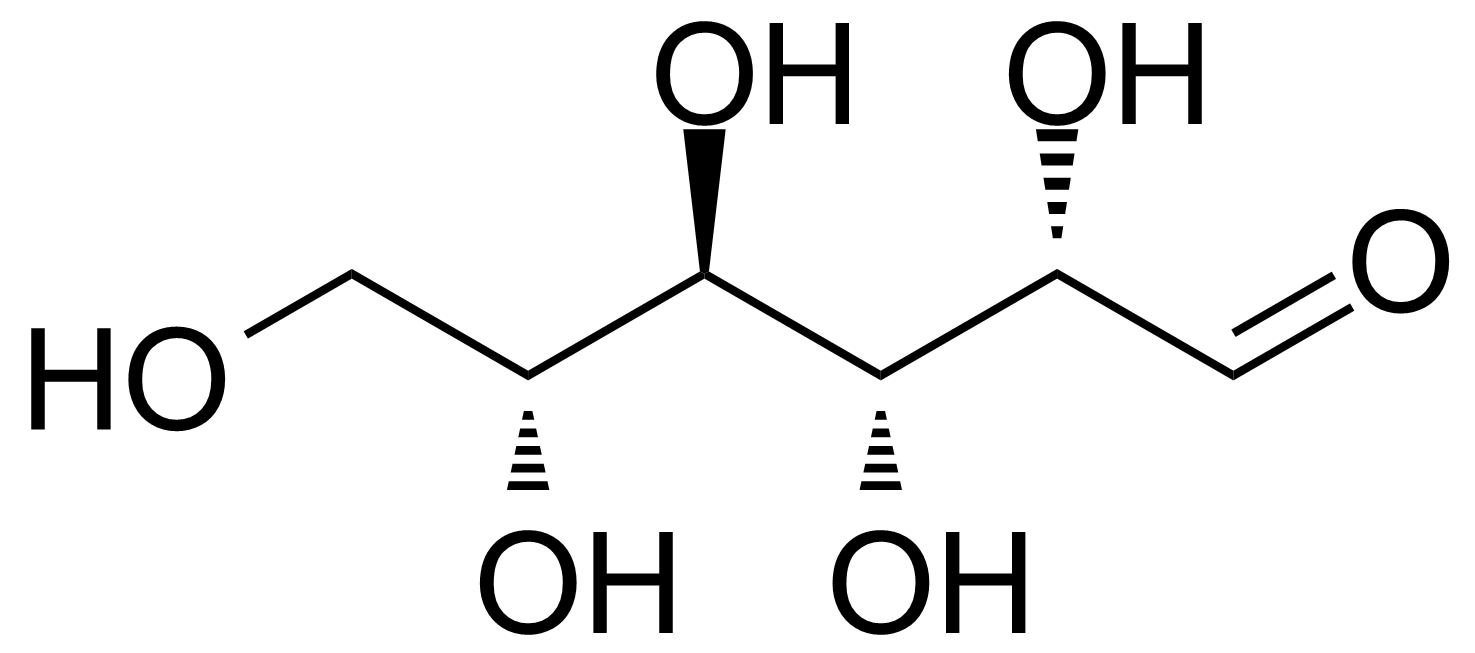 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 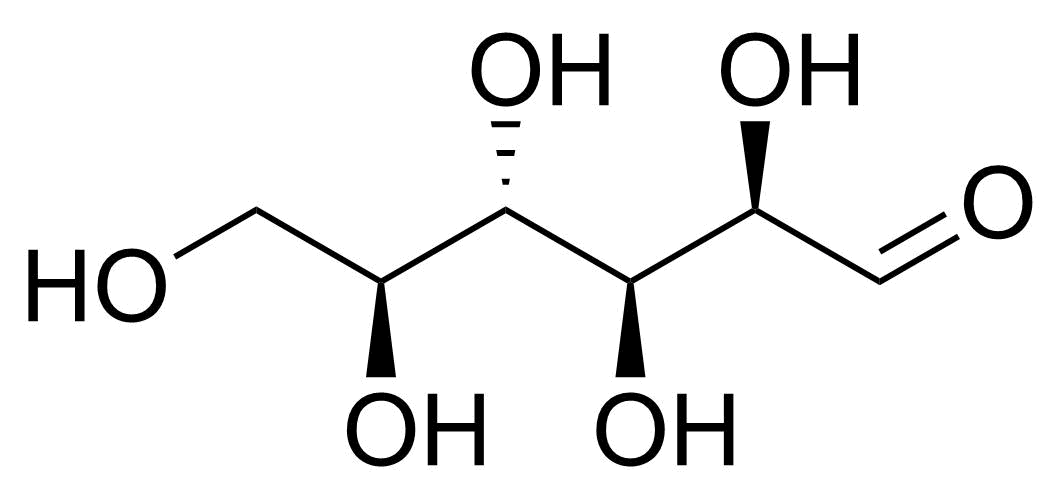 | [1949-88-8] | GEO-04662 | |
| Nouveau | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 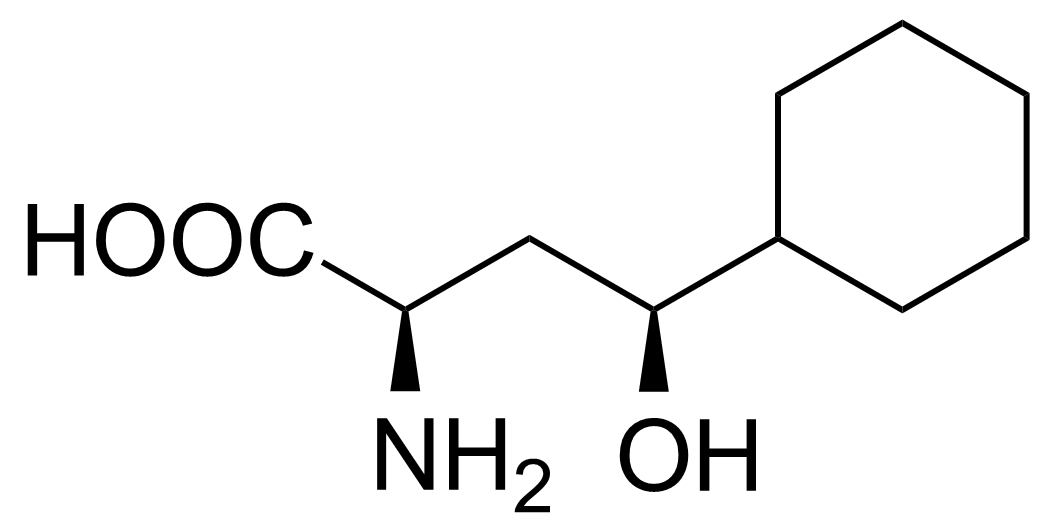 | [] | GEO-02717 |