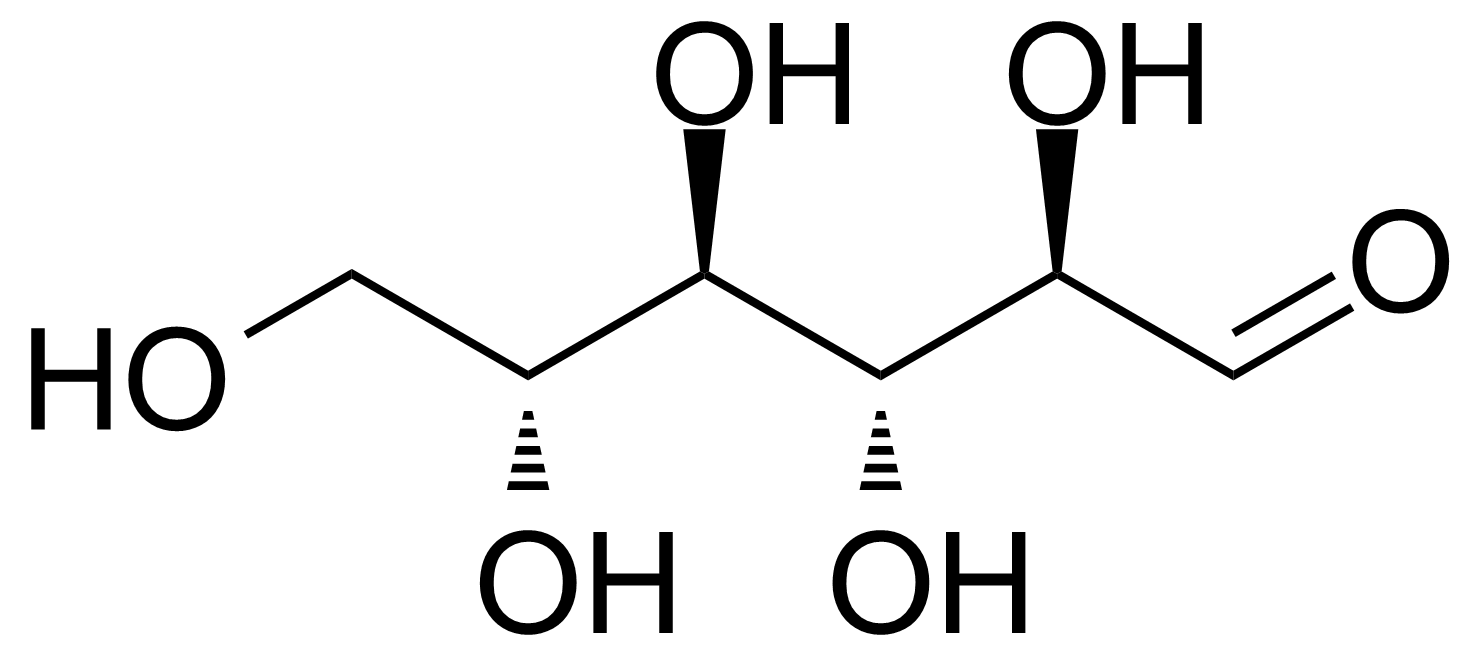
D-Allose
Ce produit n’est plus fabriqué, mais il nous reste encore un peu de stock.
Numéro CAS[2595-97-3]
G-codeGEO-00057
Numéro CE219-994-4
Formule moléculaireC6H12O6
Poids moléculaire180,16
Synonymes
a/b-D-Allopyranose
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires
Ce produit n’a pas été classé.
Catégorisation des produits
Catégorie principale
Deuxième niveau
Troisième niveau
Description
D-Allose est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des D-Allose de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of D-Allose:
D-Allose (CPA) or β-D-Allopyranose [2595-97-3] is an aldohexose monosacharid, C-3 epimer of glucose. Pure comound is colorless (white) crystalline solid with the melting point of 141-142 °C[1] and a rotation at equilibrium [α] +14.5° in water.[2] It is classified as rare sugar. D-Allose is a low-calorie sweetener (20% less sweetness than sucrose), non-toxic and it can be easily dissolved in water but it is practically insoluble in methanol. It can be found in various plant species, such as Protea rubropilosa, Veronica filiformis, Mantzelia spp. Solanum tuberosum and others.[3] Convenient laboratory preparation is based on conversion of stereochemistry of C3-hydroxy group of 1,2,5,6-di-O-isopropylidene-α-D-glucofuranose by chemical oxidation to ketone and subsequent stereospecific reduction with borohydride followed by acetals hydrolysis with Amberlite H+ resine.[2] Large scale bioproduction strategy consists of enzymatic isomerisation of D-psicose (formed biochemically from D-fructose) to D-allose, catalysed by enzyme L-rhamonose isomerase.[4] Authors proposed the bioproduction strategy route to all rare sugars in a ring form as „Izumoring“.[5]Application:
Cancer and tumor inhibition is considered to be the most important property of D-allose. It has been reported to be effective against various human cancers.[6] Furthermore, radiation, when coupled with D-allose, stimulates the production of reactive oxygen species in cancer cells to a significantly higher extent and has an approximately five times larger effect on apoptosis.[7] It also acts as an immunosuppressant[8] and due to its anti-inflammatory effect, D-allose can also mitigate cisplatin-induced nephrotoxicity.[9]Product categorization (Chemical groups):
Main category: Second level: Third level: ______________________________________________________________________________________ [1] M. L. Wolfrom, J. N. Schumacher, H. S. Isabell, F. L. Humoller J. Am. Chem. Soc. 1954, 76, 5816. [2] D.C. Baker, D. Horton, Ch. G. Tindall Jr. Carbohydr. Res. 1972, 24, 192. [3] N. Mijailovic, A. Nesler, M. Perazzolli, E. A. Barka, A. Aziz Molecules 2021, 26, 1720. [4] K. Morimoto, C. Park, M. Ozaki, K. Takeshita, T. Shimonishi, T. T. Granstrom, G. Takata, M. Tokuda, L. Izumori Enzyme Microb. Tech. 2006, 38, 855. [5] T. B. Granstrom, G. Takata, M. Tokuda, K. Izumori J. Biosci. Bioeng. 2004, 97, 89. [6] Z. Chen, J. Chen, W. Zhang, T. Zhang, C. Guang, W. Mu Appl. Microbiol. Biotechnol. 2018, 102, 426. [7] H. Hoshikawa, K. Indo, T. Mori, N. Mori Cancer Lett. 2011, 306, 60. [8] M.A. Hossain, H. Wakabayashi, F. Goda, S. Kobayashi, T. Maeba, H. Maeta Transplant. Proc. 2000, 32, 2021. [9] Y. Iga, T. Matsuo J. Jpn. Soc. Nutr. Food Sci. 2010, 63, 17.
Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
| Nouveau | 1-Acenaphthenol | 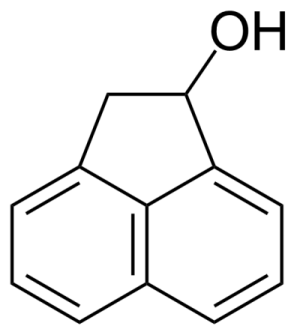 | [6306-07-6] | GEO-00001 |
| Nouveau | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 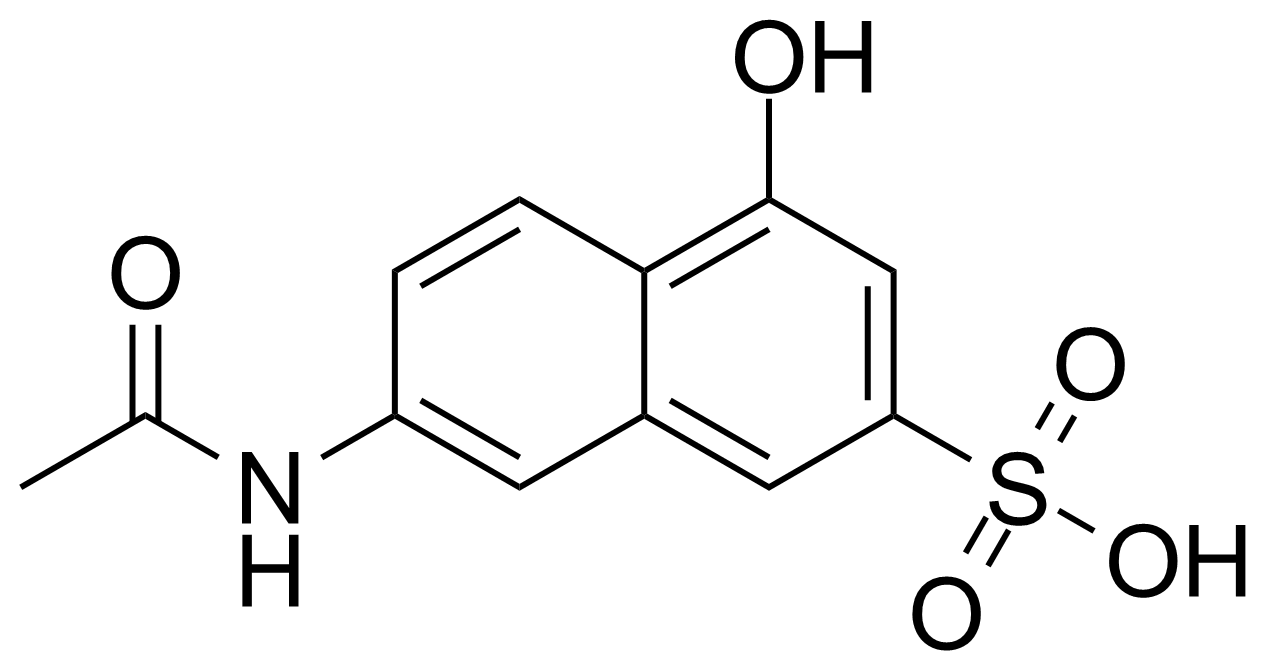 | [6334-97-0] | GEO-04013 |
| Nouveau | 1-Adamantanemethanol | 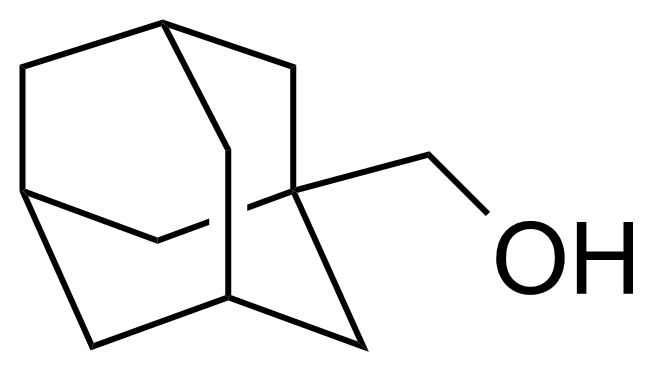 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 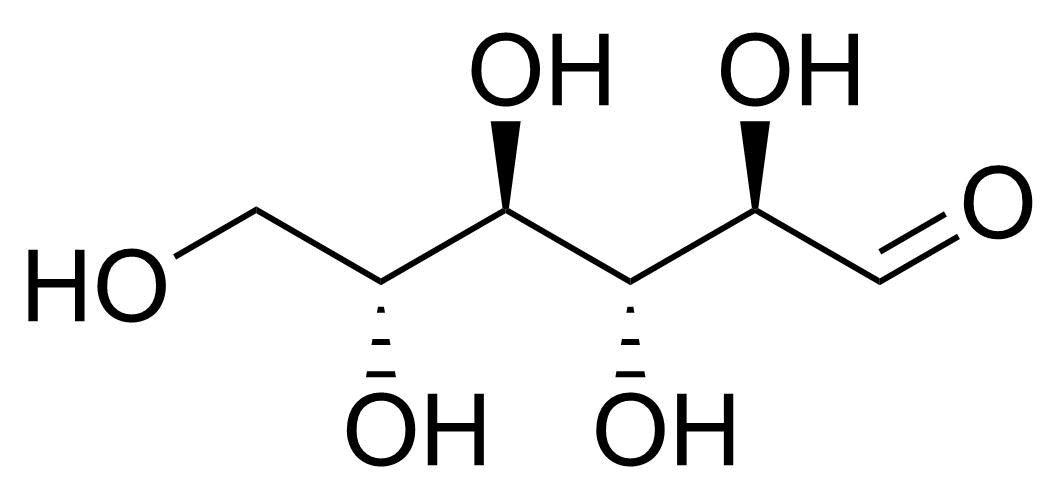 | [7283-09-2] | GEO-04660 | |
| Nouveau | D-Allose |  | [2595-97-3] | GEO-00057 |
| L-Allose | 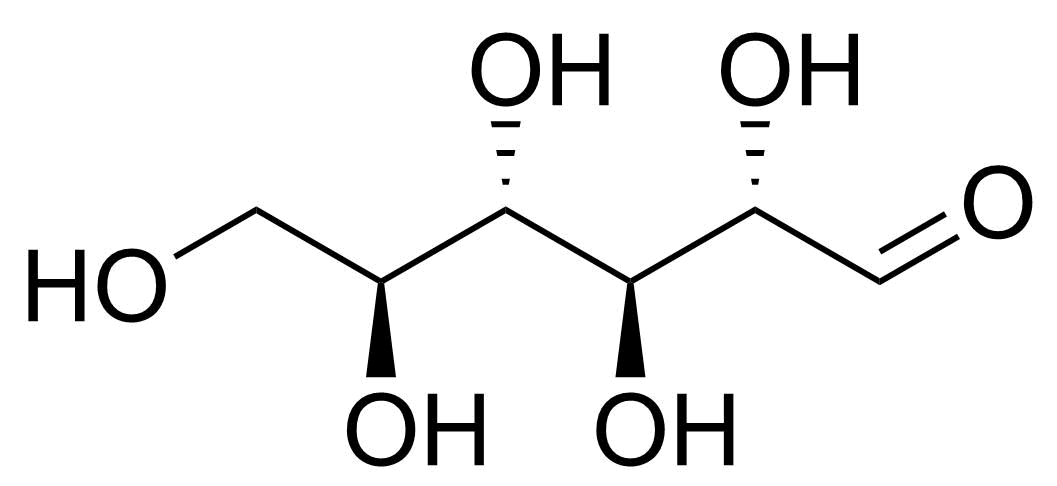 | [39392-62-6] | GEO-04661 | |
| Nouveau | 2-(Allyloxy)phenol | 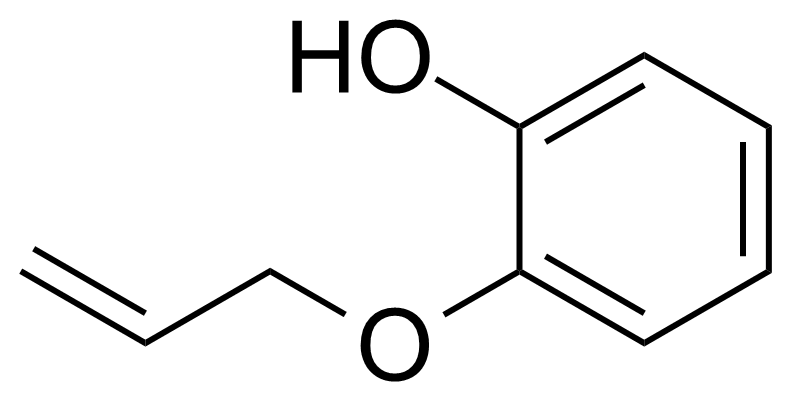 | [1126-20-1] | GEO-04471 |
| D-Altrose | 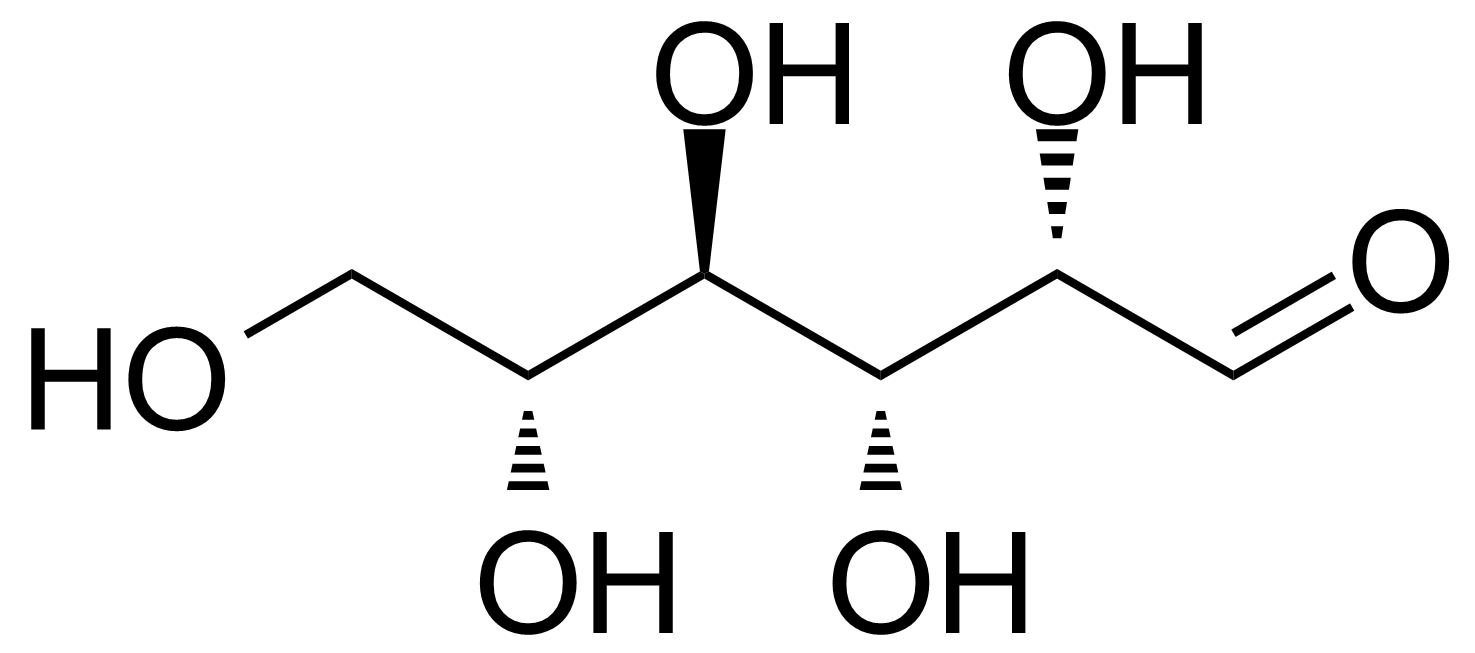 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 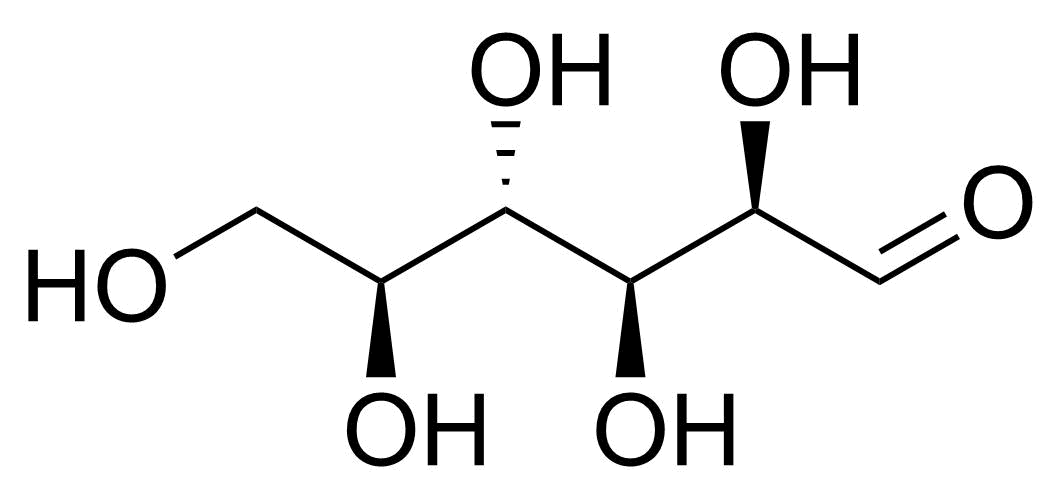 | [1949-88-8] | GEO-04662 | |
| Nouveau | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 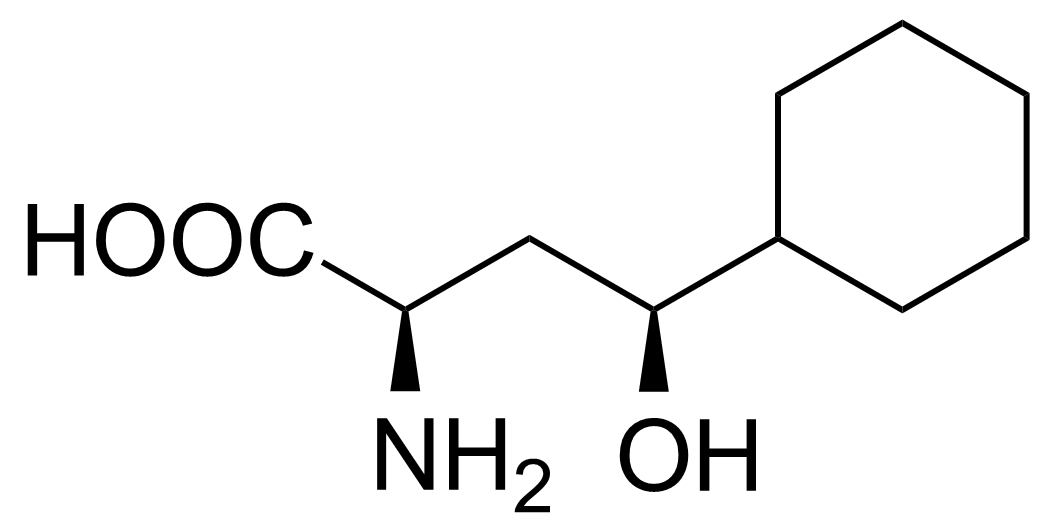 | [] | GEO-02717 |