Cyclobutanol
CAS-Nr.[2919-23-5]
G-CodeGEO-03202
EC-Nummer220-858-1
SummenformelC4H8O
Molekulargewicht72,11
Für weitere Informationen oder eine Anfrage senden Sie uns bitte eine E-Mail oder nutzen Sie unser Kontaktformular
Regulatorische Informationen
Dieses Produkt ist nicht klassifiziert.
Produktkategorisierung
Hauptkategorie
Zweite Ebene
Beschreibung
Cyclobutanol ist eine nützliche chemische Verbindung mit vielfältigen Forschungsanwendungen. Wir freuen uns, qualitativ hochwertige Cyclobutanol in verschiedenen Größen (für Forschungs-, Pilotmaßstabs- oder Produktionsanwendungen) von Milligramm- bis Multi-Kilogramm-Chargen anbieten zu können, sodass Sie ganz einfach die richtige Menge für Ihre Bedürfnisse auswählen können.
Vollständige Beschreibung anzeigenUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of Cyclobutanol:
Cyclobutanol, or cyclobutyl alcohol or hydroxycyclobutane [2919-23-5] is an organic compound belonging to the class of alcohols. It is a colorless liquid with the boiling point of 122-124 °C.[1] Cyclobutanol and its vapors are highly flammable. It is only partially miscible in water, and readily soluble in common organic solvents. It can be prepared from cyclopropanemethanol by refluxing with aqueous hydrochlorid acid. Crude product is purified by distillation from 3-buten-1-ol as byproduct.[1] Another convenient option for the preparation is the reduction of cyclobutanone with lithium aluminium hydride.[2] Cyclobutanone can be prepared by the reaction of diazomethane with ketene in diethyl ether.[3]Application:
Cyclobutanol is a valuable reagent in synthetic chemistry. It was used in the preparation of tripeptide derivative as potent ACE inhibitor.[4] 1-Arylsubstituted cyclobutanols undergo silver-catalyzed ring opening forming the open-chain alkyl radical which can be either trapped by SelectFluor to furnish γ-fluorinated ketones.[5] The reaction without extrinsic radical scavenger gives the 1-tetralones via intramolecular cyclization.[6] Cyclobutanol ring expansion to 1-tetralones can be induced also electrochemically.[7] Alkenyl cyclobutanols can be used in catalyst-free selenylation/semipinacol rearrangement cascades to furnish β-selenylated cyclopentanones.[8]Product categorization (Chemical groups):
Main category: Second level: ______________________________________________________________________________________ [1] J. Salaün, A. Fadel Org. Synth. 1986, 64, 50. [2] J. D. Roberts, Ch. W. Sauer J. Am. Chem. Soc. 1949, 71, 3925. [3] P. Lipp, R. Köster Ber. Dtsch. Chem. Ges. 1931, 64, 2823. [4] T. Sawayama, M. Tsukamoto, T. Sasagawa, K. Nishimura, T. Defgughi, K. Takeyama, K. Hosoki Chem. Pharm. Bull. 1990, 38, 110. [5] H. Zhao, X. Fan, J. Yu, Ch. Z. J. Am. Chem. Soc. 2015, 137, 3490. [6] J. Yu, H. Zhao, S. Liang, X. Bao, Ch. Zhu, Org. Biomol. Chem. 2015, 13, 7924. [7] A. Petti, P. Natho, K. Lam, P. J. Parsons Eur. J. Org. Chem. 2021, 854. [8] D. Y. Kim Synth. Commun. 2019, 49, 2203.
Ähnliche Produkte
| Produktname | Struktur | CAS-Nr. | G-Code | |
|---|---|---|---|---|
| Neu | 1-Acenaphthenol | 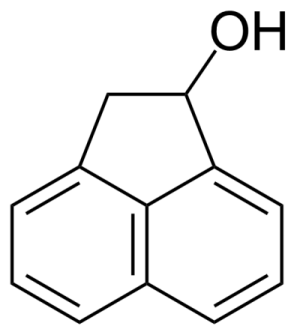 | [6306-07-6] | GEO-00001 |
| Neu | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 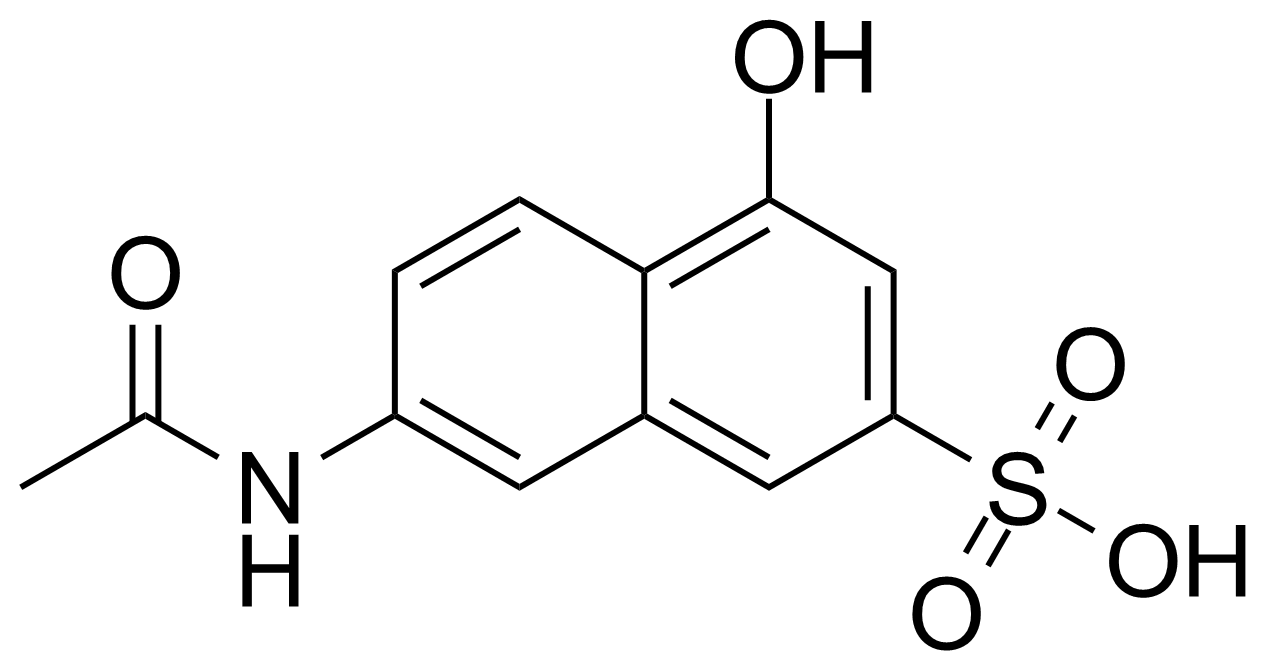 | [6334-97-0] | GEO-04013 |
| Neu | 1-Adamantanemethanol | 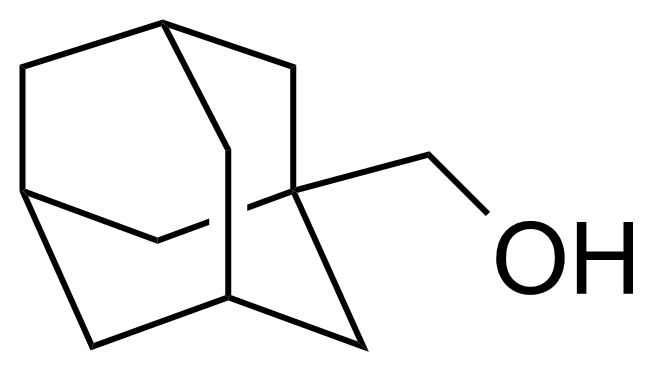 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 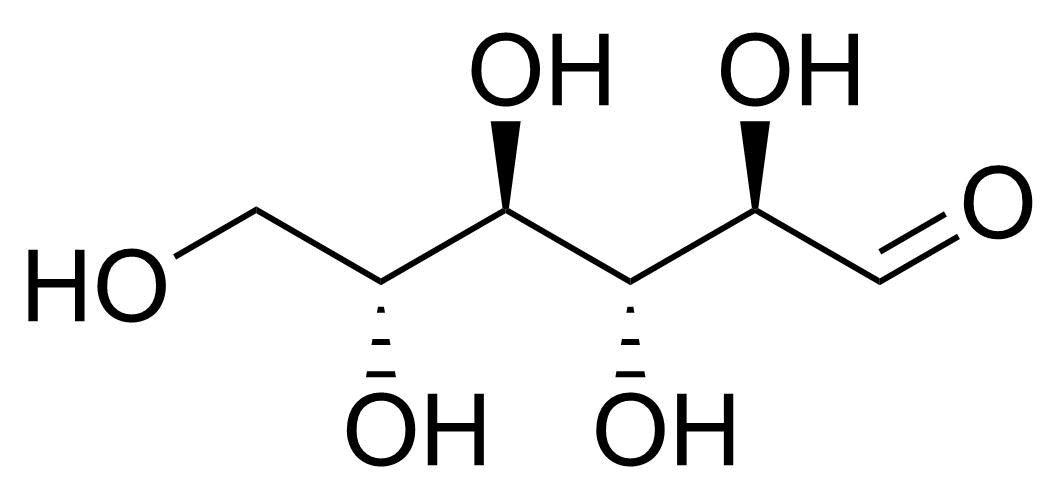 | [7283-09-2] | GEO-04660 | |
| Neu | D-Allose | 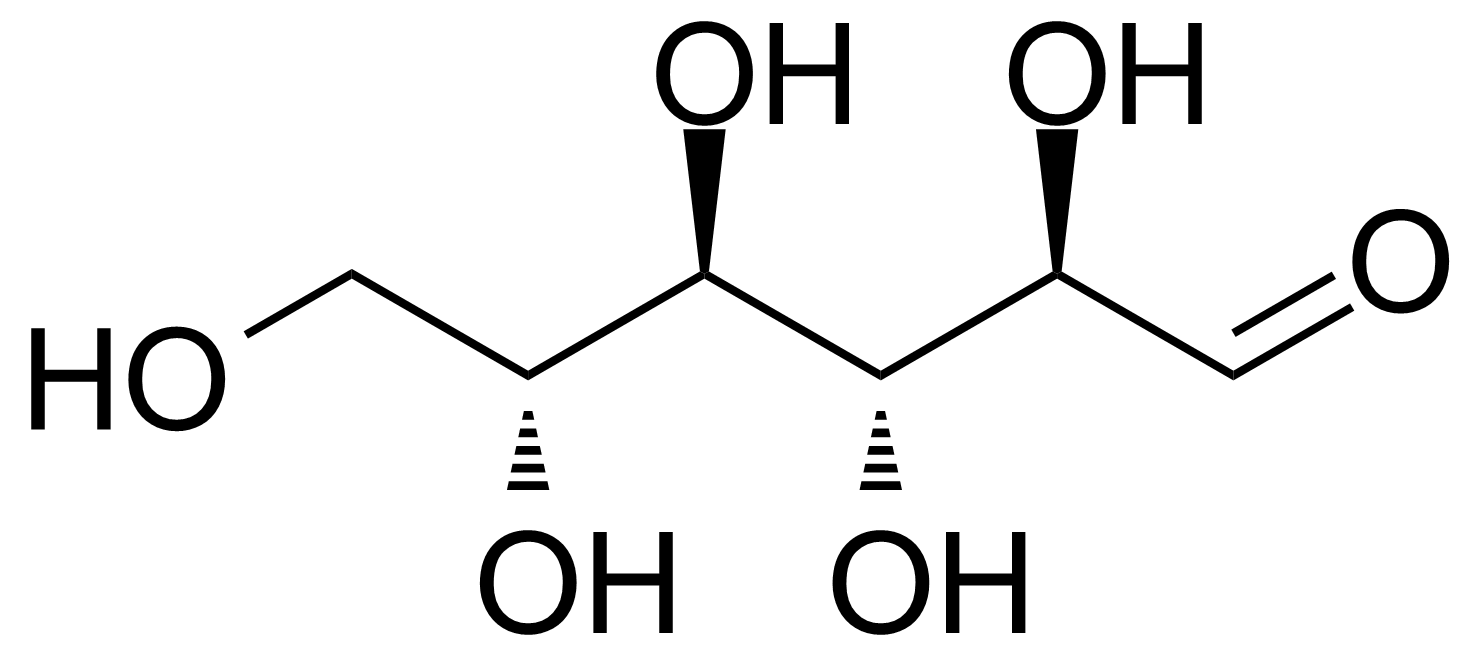 | [2595-97-3] | GEO-00057 |
| L-Allose | 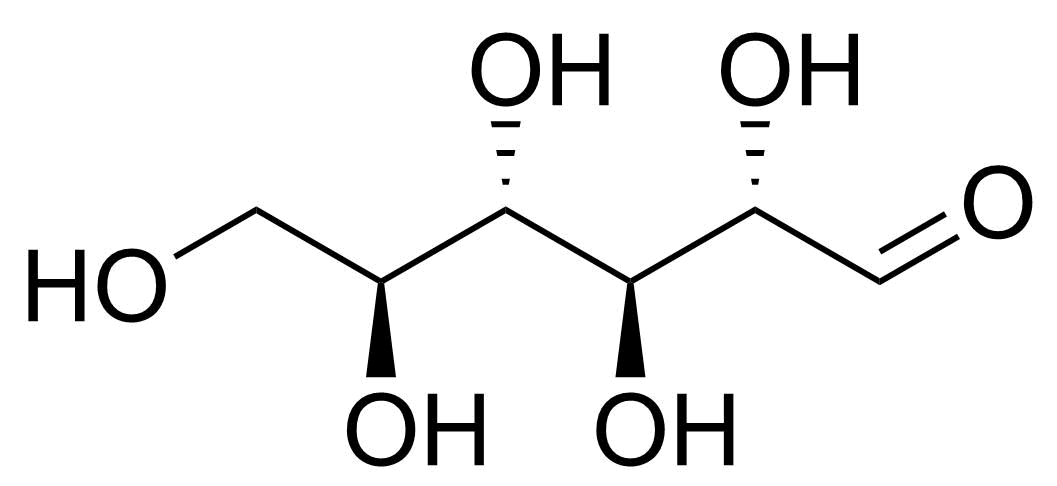 | [39392-62-6] | GEO-04661 | |
| Neu | 2-(Allyloxy)phenol | 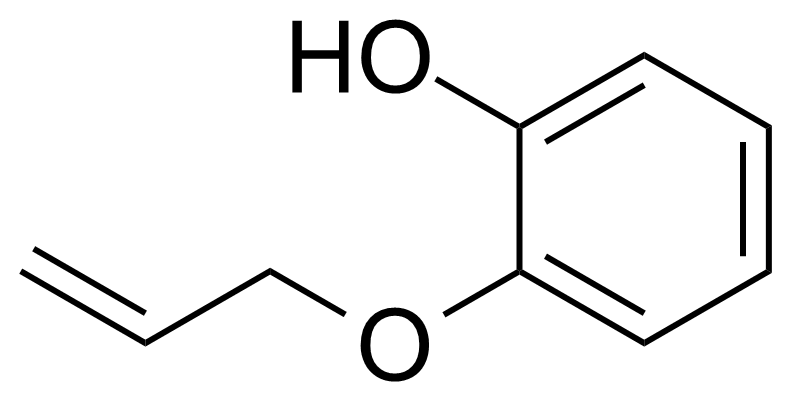 | [1126-20-1] | GEO-04471 |
| D-Altrose | 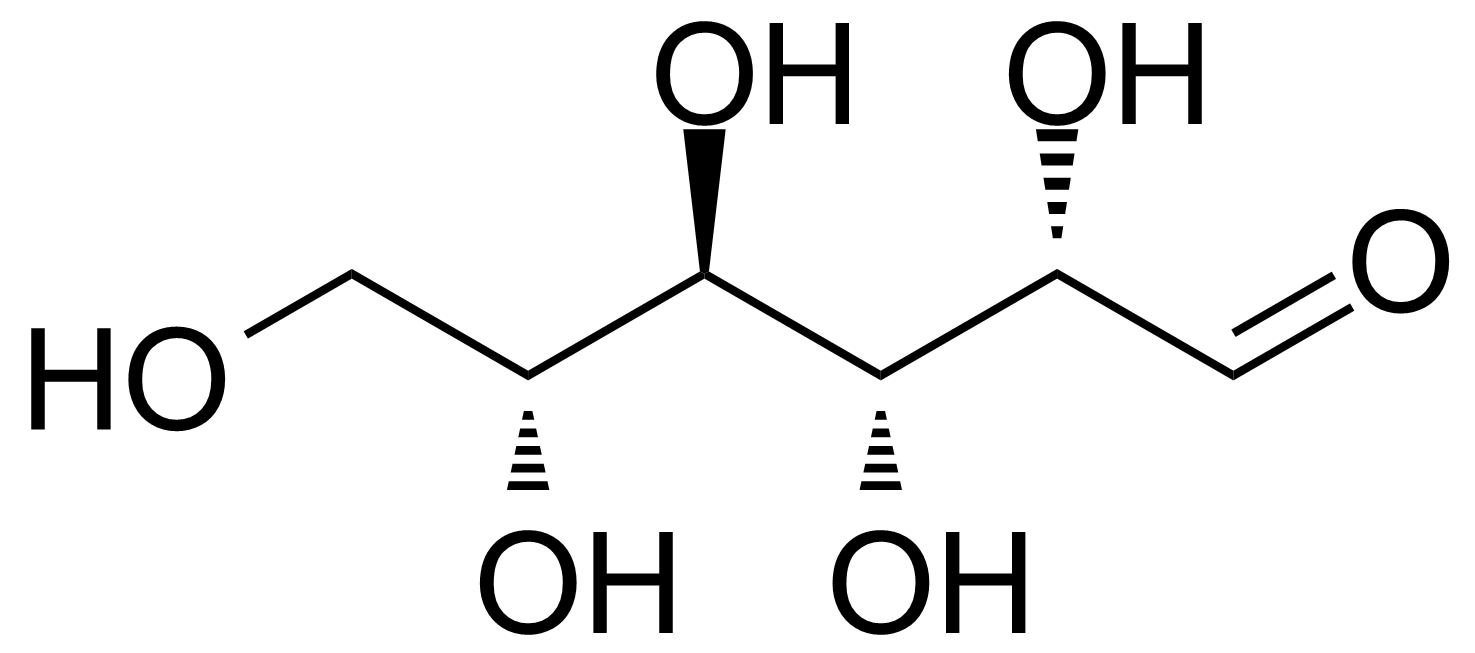 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 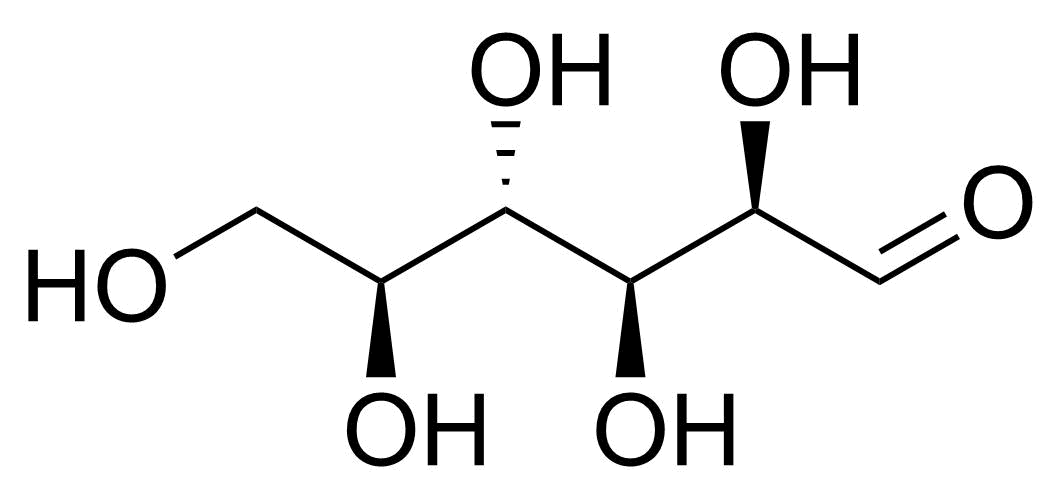 | [1949-88-8] | GEO-04662 | |
| Neu | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 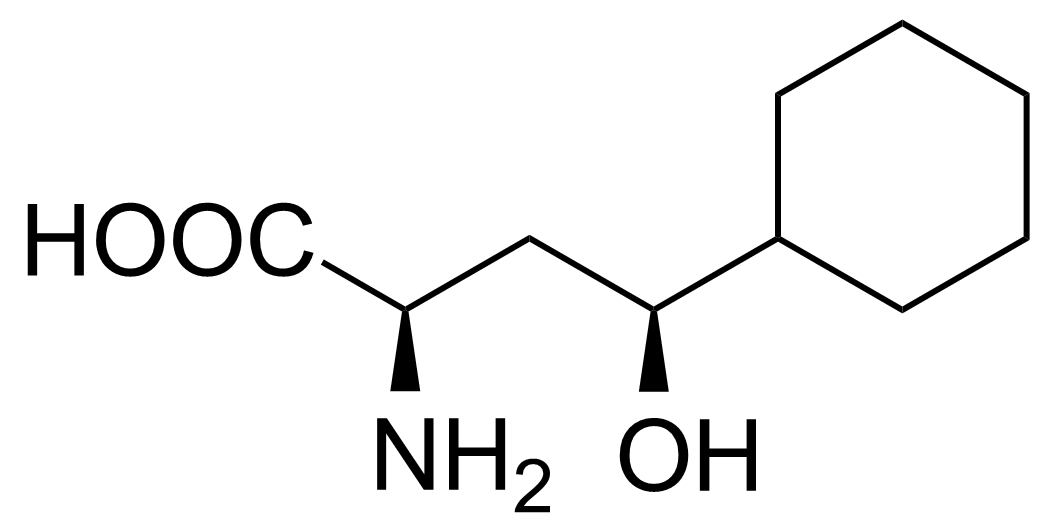 | [] | GEO-02717 |