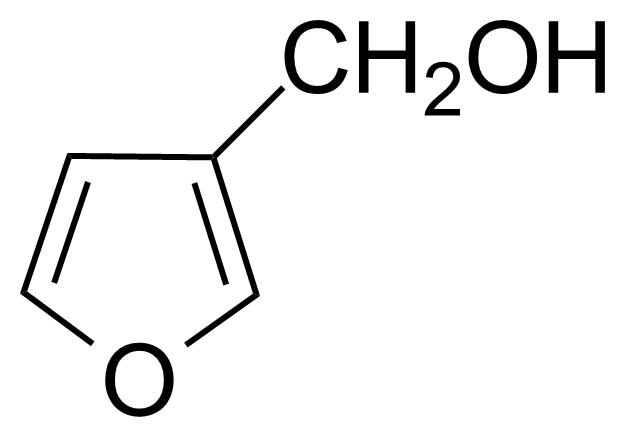
Furan-3-methanol
3-(Hydroxymethyl)furan ; 3-Furanmethanol ; 3-Furancarbinol ; 3-Furfuryl alcohol ; 3-Furylcarbinol
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information


H226 – Flammable liquid and vapour
H302 – Harmful if swallowed
H312 – Harmful in contact with skin
H332 – Harmful if inhaled
P210 – Keep away from heat/sparks/open flames/hot surfaces – No smoking:
P261 – Avoid breathing dust/fume/gas/mist/vapours/spray:
P280 – Wear protective gloves/protective clothing/eye protection/face protection:
P301+312 – IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell
P302+352 – IF ON SKIN: Wash with soap and water
P304+340 – IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+351+338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing
Product categorization
Description
Furan-3-methanol is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Furan-3-methanol in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Show full descriptionPreparation of Furan-3-methanol:
Furan-3-methanol can be easily obtained by the reduction of appropriate furoic aldehyde[2], acid[3] and ester[4].Application:
It is commonly used in various industries, including pharmaceuticals, resins, and coatings. Furan-3-methanol possesses unique properties, such as its ability to polymerize and form cross-linked networks, making it an essential component in the production of thermosetting resins. Additionally, it is utilized as a solvent and a flavoring agent in food and beverages. It is useful building block in organic synthesis. Furan derivative is an imperative class of heterocyclic compound that has important biological properties. For example, it was used as a starting material for the synthesis of camptothecin and its derivatives[2], various sasquiterpenes[5] and pyridazinones with biological activity[6].Product categorization (Chemical groups):
Main category: Second level: Third level: _______________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| New | 1-Acenaphthenol | 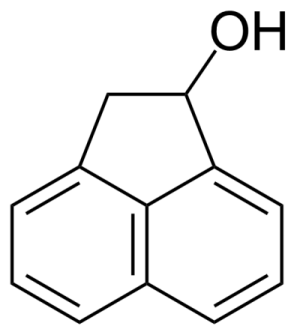 | [6306-07-6] | GEO-00001 |
| New | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 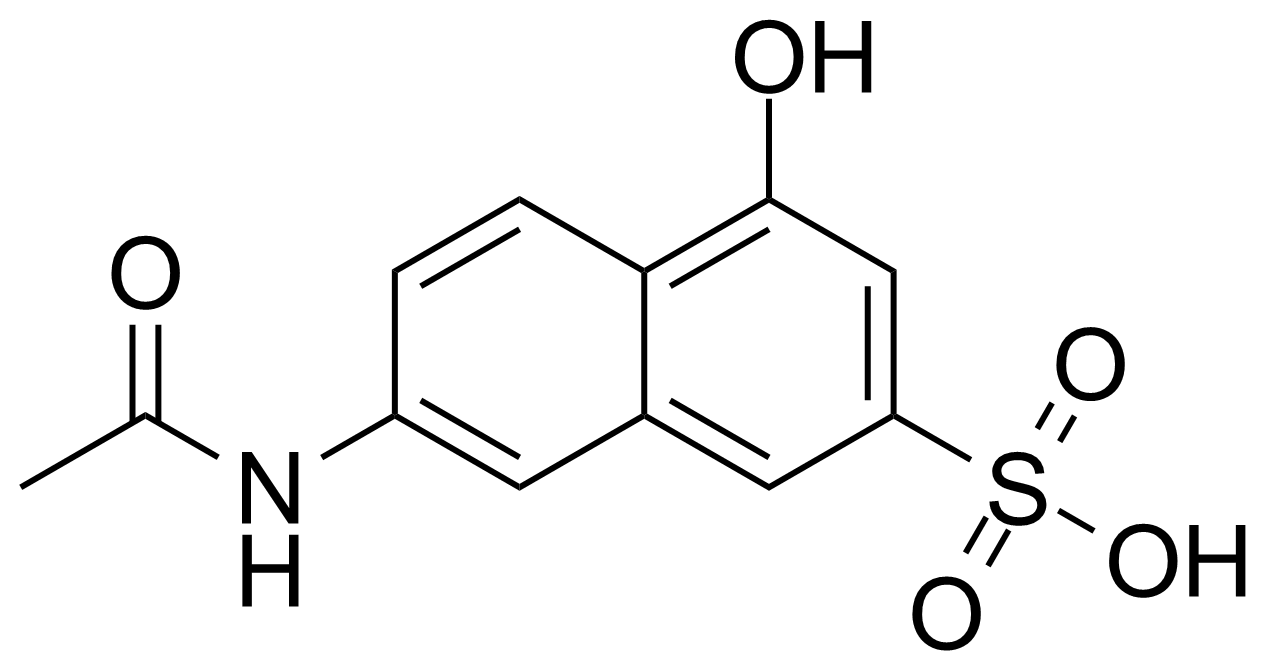 | [6334-97-0] | GEO-04013 |
| New | 1-Adamantanemethanol | 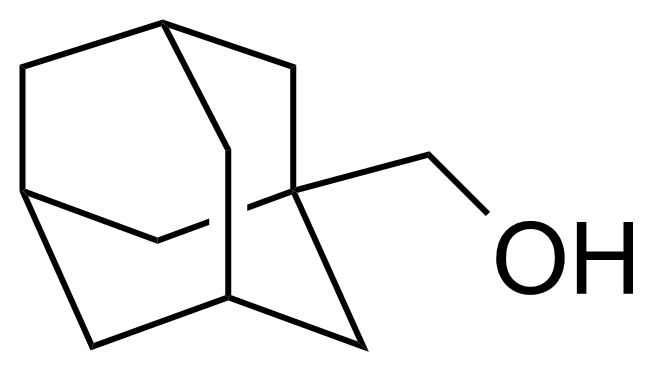 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 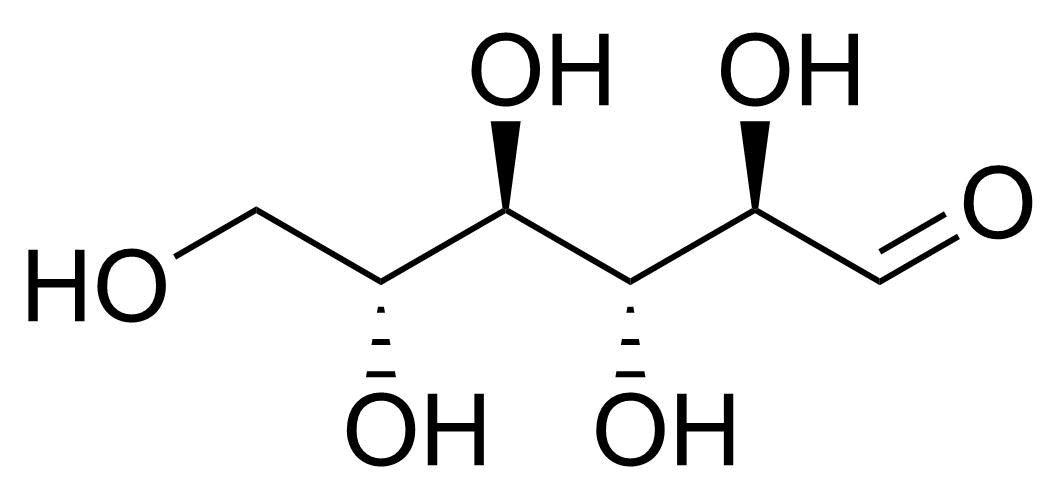 | [7283-09-2] | GEO-04660 | |
| New | D-Allose | 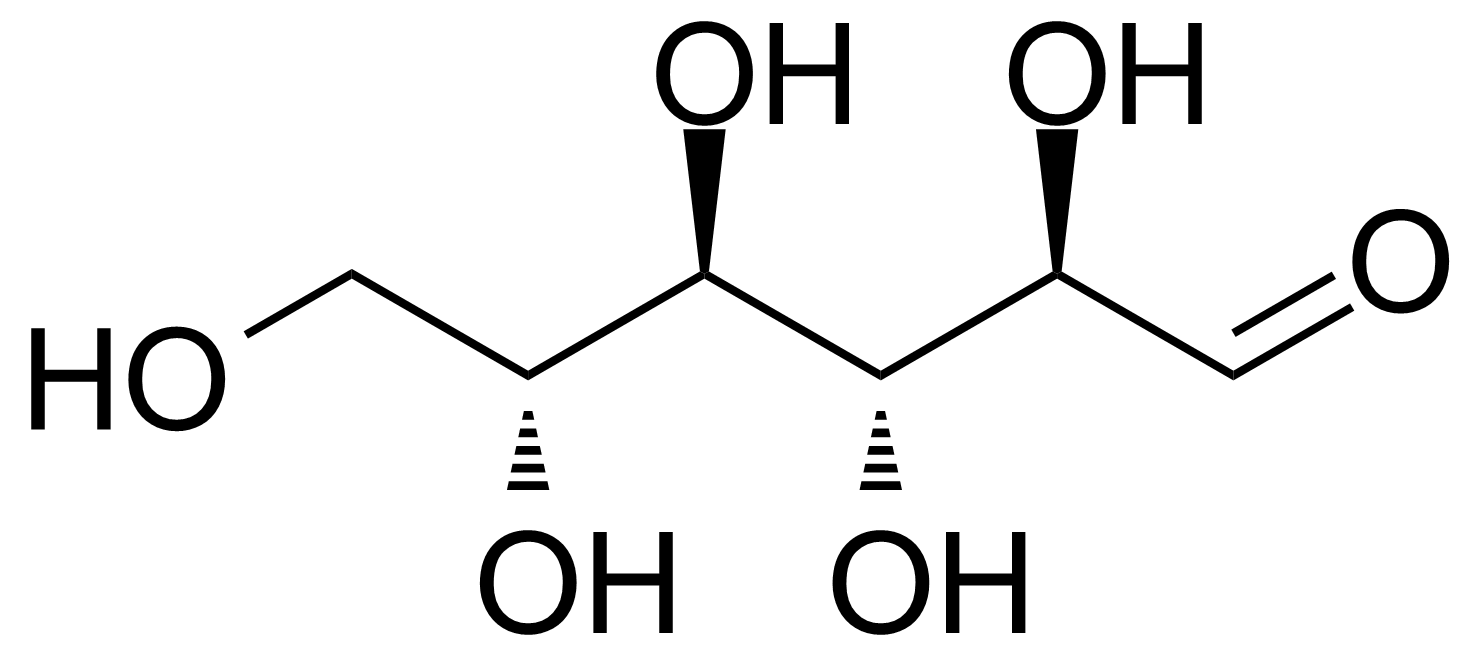 | [2595-97-3] | GEO-00057 |
| L-Allose | 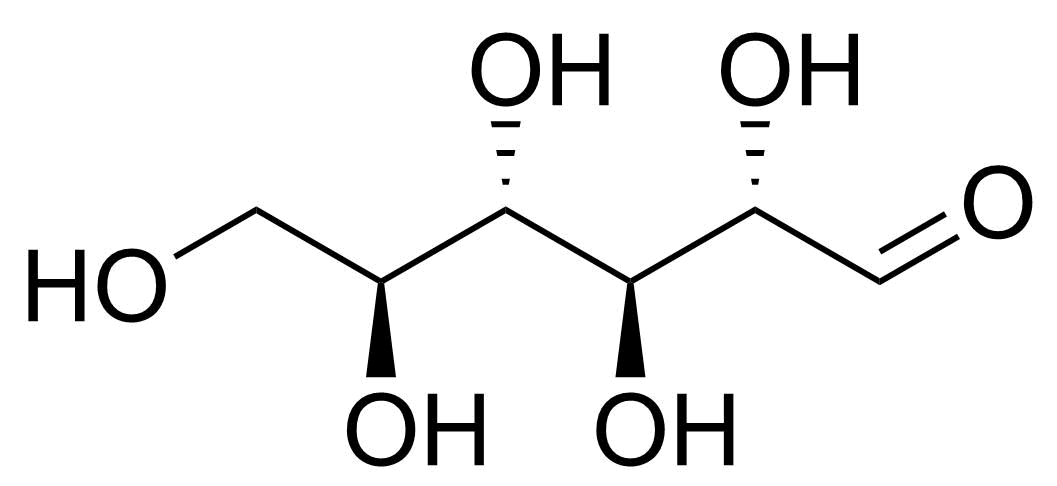 | [39392-62-6] | GEO-04661 | |
| New | 2-(Allyloxy)phenol | 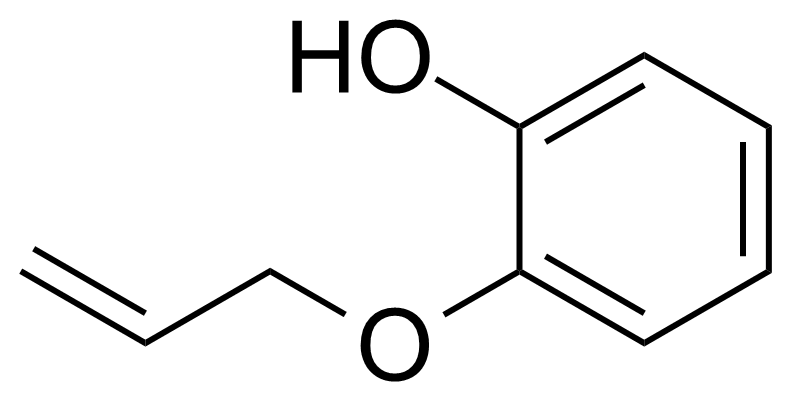 | [1126-20-1] | GEO-04471 |
| D-Altrose | 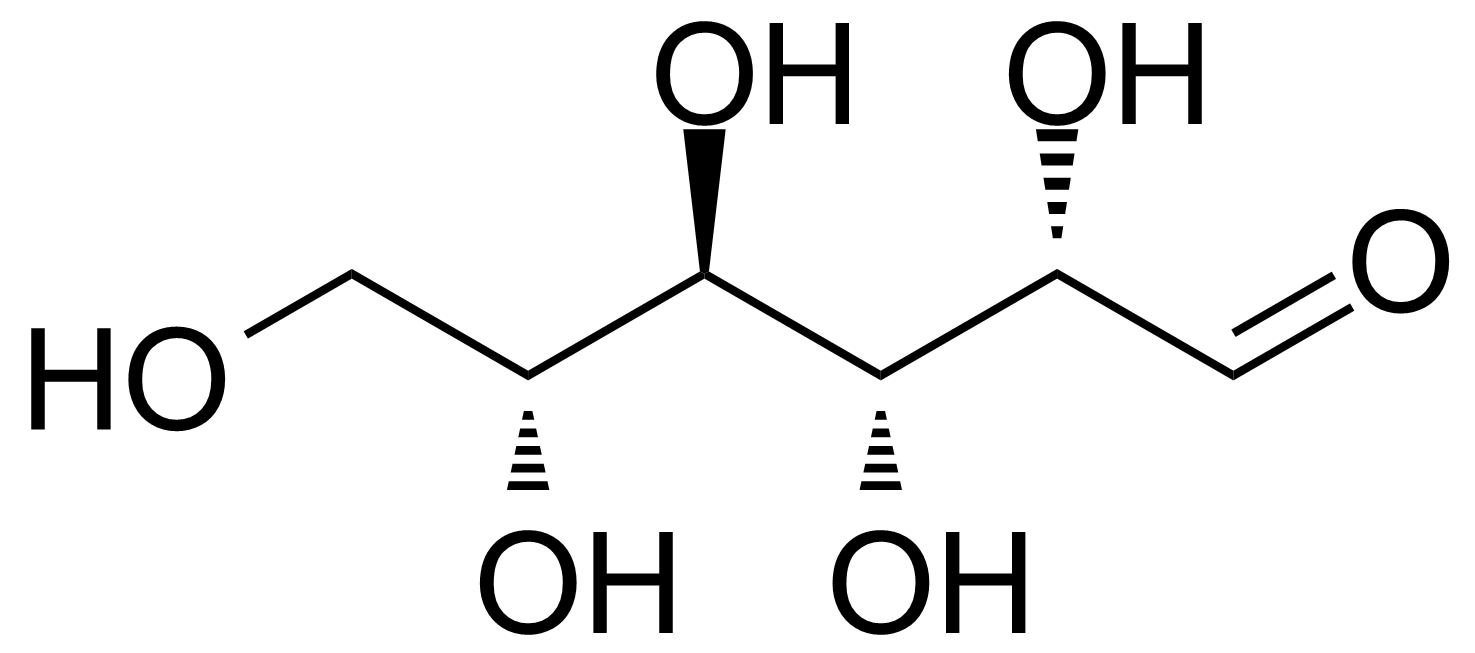 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 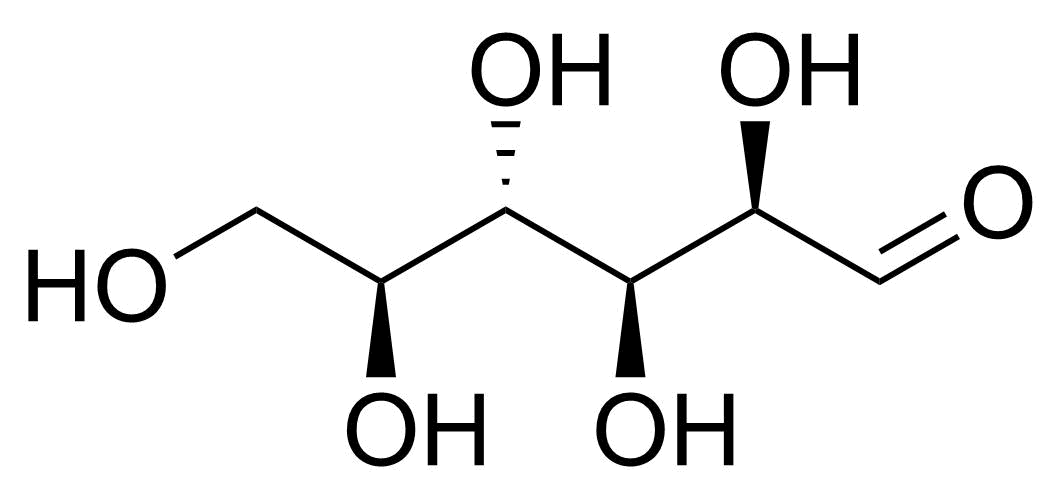 | [1949-88-8] | GEO-04662 | |
| New | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 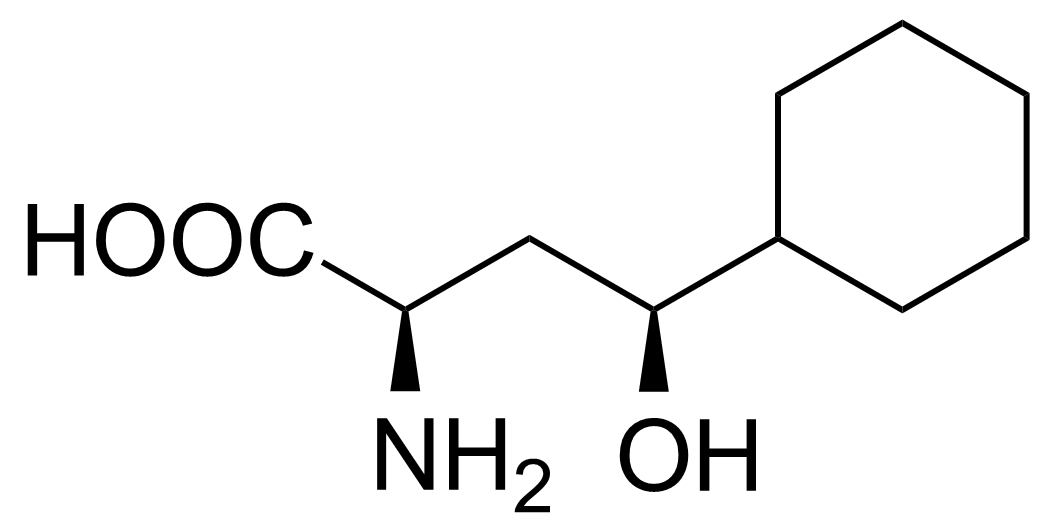 | [] | GEO-02717 |