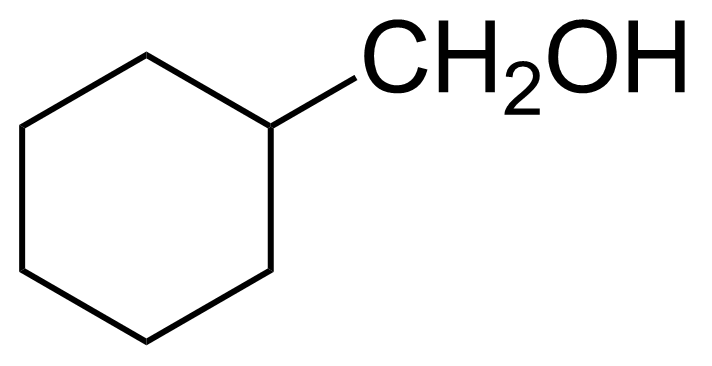
Cyclohexanemethanol
Product has been discontinued; however, we still have inventory in stock.
(Hydroxymethyl)cyclohexane ; Hexahydrobenzyl alcohol ; Cyclohexylcarbinol ; Cyclohexylmethanol
For more information or to place an inquiry, please email us to
georganics@georganics.sk or use our contact form
Regulatory Information
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
Product categorization
Description
Cyclohexanemethanol is a useful chemical compound with a variety of research applications. We are pleased to offer high quality Cyclohexanemethanol in various sizes (for research, pilot-scale, or production applications) from milligrams to multi-kilogram batches, making it easy for you to choose the right amount to suit your needs.
Cyclohexanemethanol is usually used as a reagent in organic synthesis. It acts as a nucleohpile in highly beta-selective glucosidation of…
Show full descriptionGeneral description of Cyclohexanemethanol:
Cyclohexanemethanol (CM), or (hydroxymethyl)cyclohexane, or cyclohexylcarbinol [100-49-2] is six-membered alicyclic organic compound that belong to alcohols. In its pure form, it is colorless viscious highly flammable liquid with the boiling point of 179-180 °C.[1] It is insoluble in water and soluble in common organic solvents. CM is a non-corrosive and quick volatilize liquid with the LD50 = 250 mg/kg (mouse, intraperitoneal).[2] Convenient laboratory preparation starts with chlorocyclohexane that is converted to cyclohexylmagnesium chloride, which reacts with formaldehyde or paraformaldehyde.[3]Application of Cyclohexanemethanol:
Cyclohexanemethanol is usually used as a reagent in organic synthesis. It acts as a nucleohpile in highly beta-selective glucosidation of ethylthioglucosides.[4] It can be used as a starting material for the synthesis of cyclohexanecarboxaldehyde, cyclohexanecarboxylic acid, cyclohexanone, and 1,4-cyclohexadione by photocatalytic oxidation using titanium dioxide nanoparticles.[5] Cyclohexanemethanol can be biologically oxidized by the yeasts Candida maltosa and Trichosporon mucoides under laboratory conditions.[6]Important Notes:
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible materials are Strong oxidizing agents, Acid chlorides, Acid anhydrides. Excess heat. Keep away from open flames, hot surfaces and sources of ignition. Hazardous Combustion Products: Carbon monoxide (CO), Carbon dioxide (CO2). Cyclohexanemethanol is stable under normal conditionsProduct categorization (Chemical groups):
Main category: Second level: ______________________________________________________________________________________Similar products
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| New | 1-Acenaphthenol | 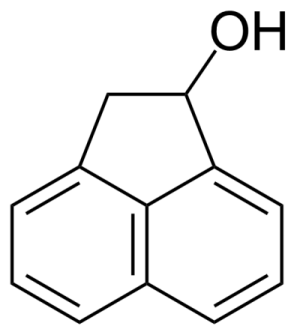 | [6306-07-6] | GEO-00001 |
| New | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 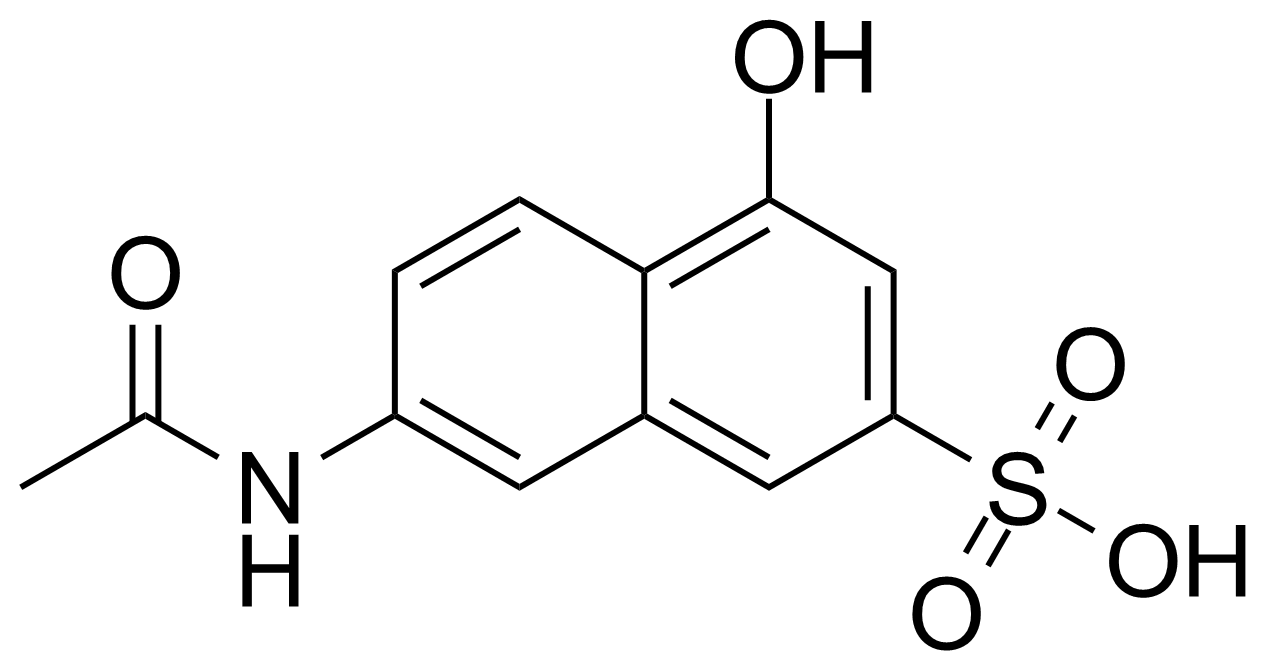 | [6334-97-0] | GEO-04013 |
| New | 1-Adamantanemethanol | 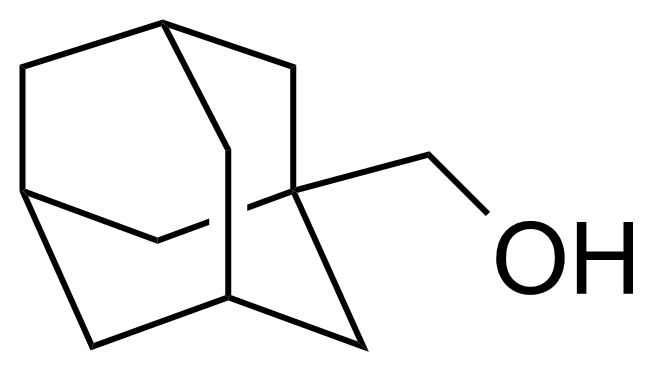 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 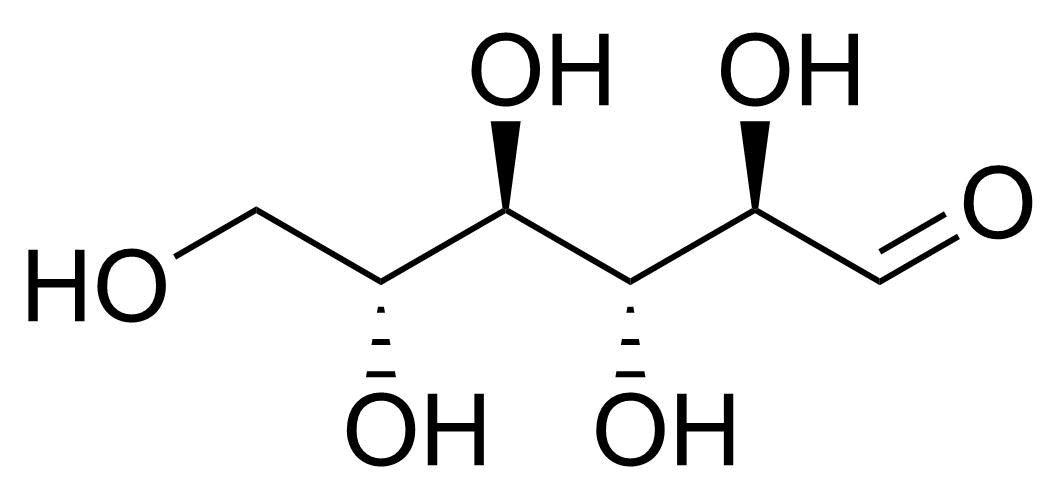 | [7283-09-2] | GEO-04660 | |
| New | D-Allose | 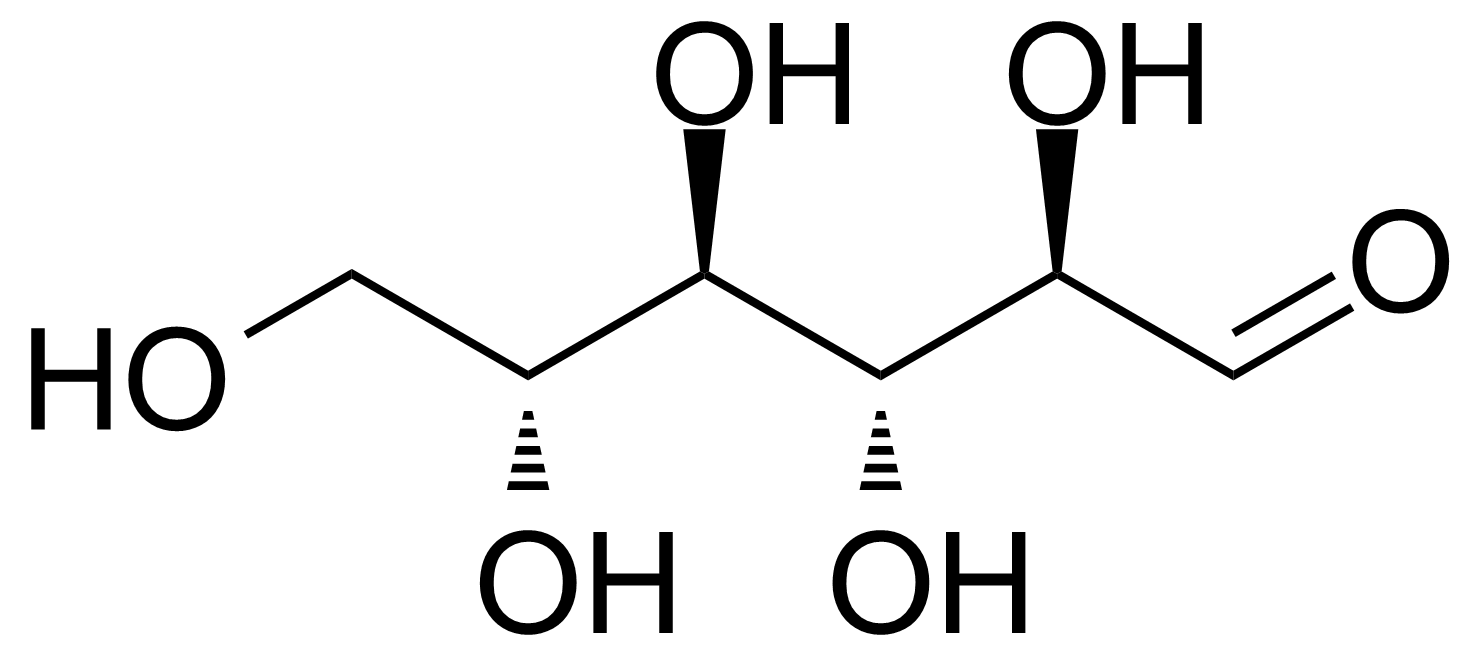 | [2595-97-3] | GEO-00057 |
| L-Allose | 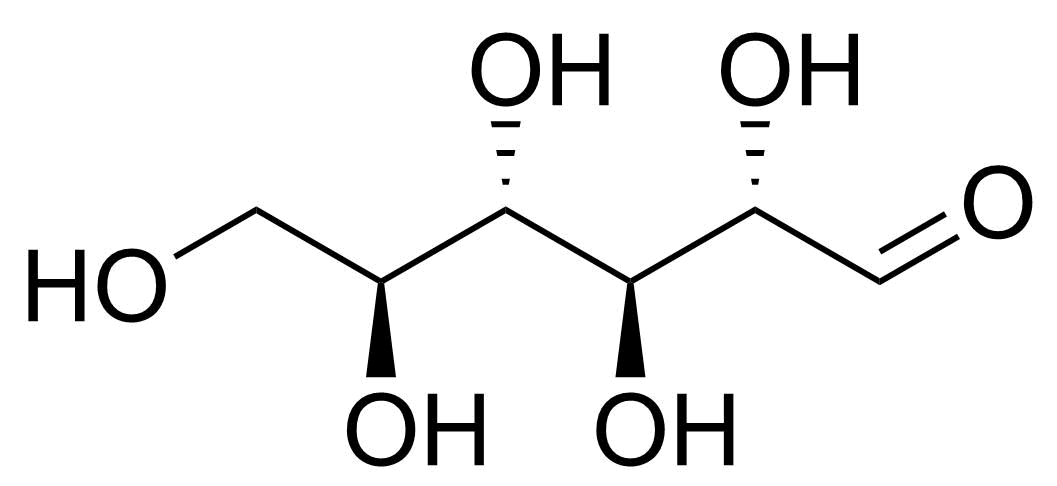 | [39392-62-6] | GEO-04661 | |
| New | 2-(Allyloxy)phenol | 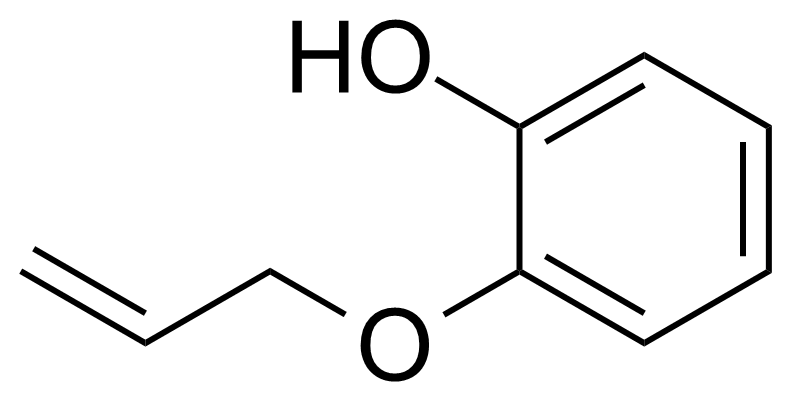 | [1126-20-1] | GEO-04471 |
| D-Altrose | 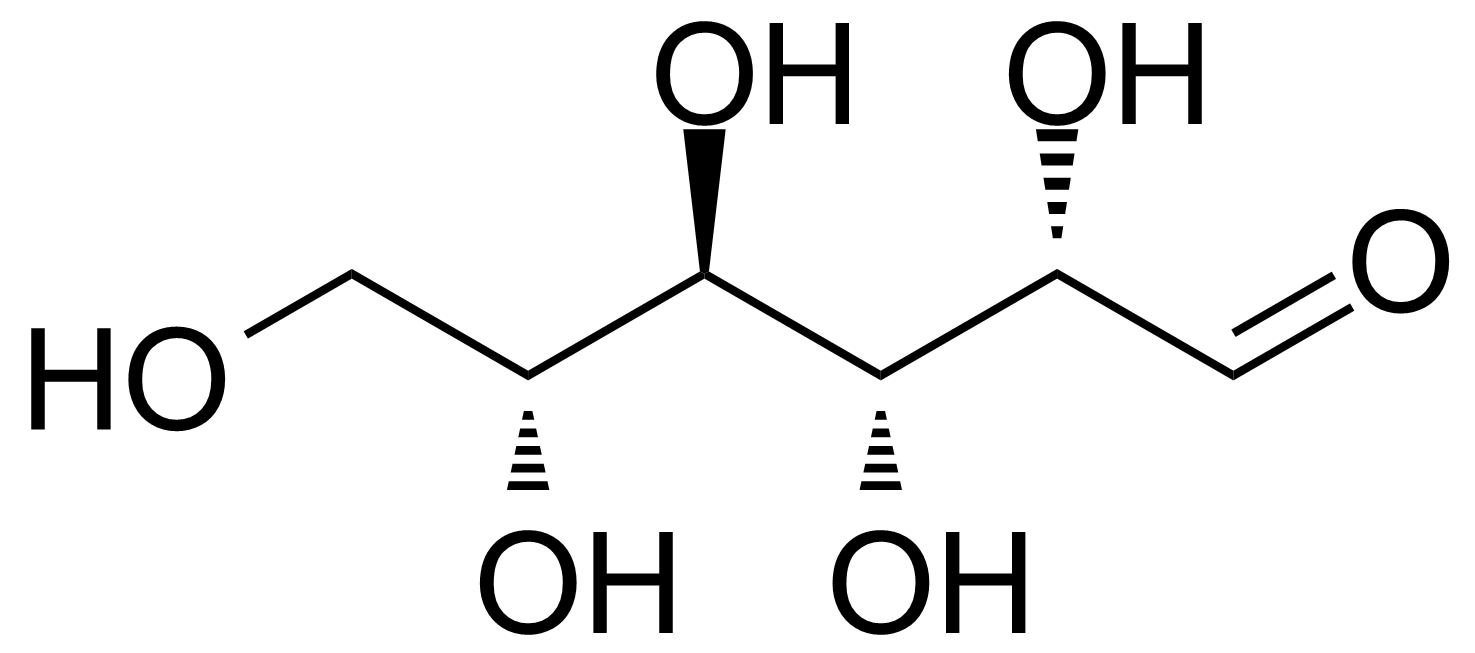 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 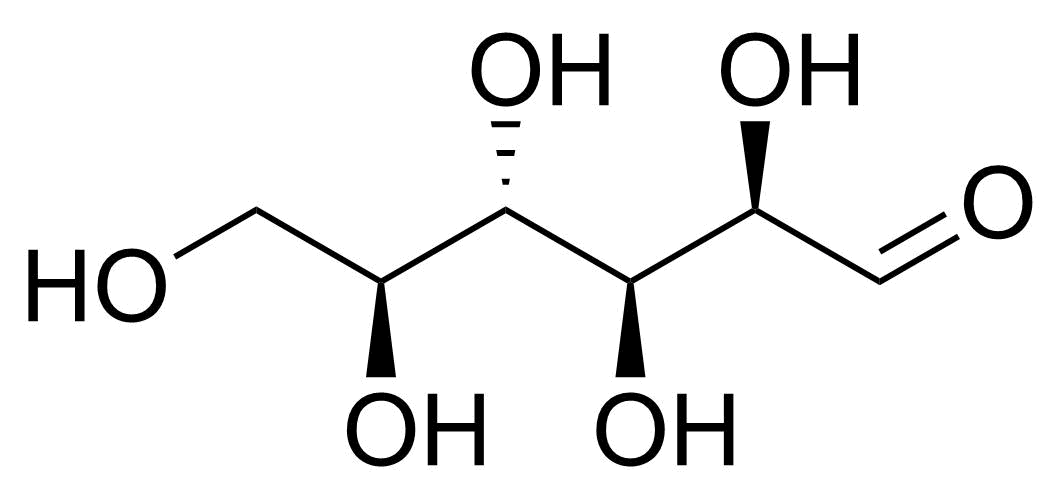 | [1949-88-8] | GEO-04662 | |
| New | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 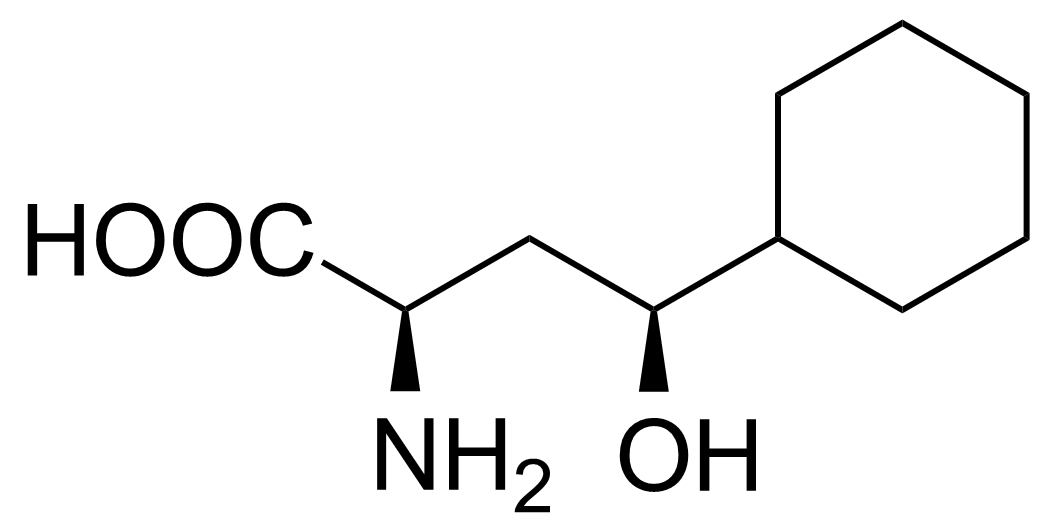 | [] | GEO-02717 |