Sulfoxides / Sulfones
Sulfoxides / Sulfones are a group of organosulfur compounds formed by oxidation of appropriate sulfides via sulfoxides to sulfones. Sulfoxides contain sulfinyl group while sulfones contain sulfonyl group. Sulfones can be also prepared under Friedel–Crafts reaction conditions using sulfonyl halides and sulfonic acid anhydrides or by nucleophilic displacement of halides by sulfinates. Sulfoxides undergo deoxygenation back to sulfides. Alkyl sulfoxides react with acetic anhydride to give migration of the oxygen from sulfur to the adjacent carbon as an acetate ester (Pummerer rearrangement). Sulfoxides can undergo thermal elimination via an Ei mechanism to yield vinyl alkenes.
In the Ramberg–Bäcklund reaction and the Julia olefination, sulfones are converted to alkenes. Examples of important naturally occurring chiral sulfoxides are alliin and ajoene, both present in garlic, and dimethyl sulfoxide (DMSO), a common solvent. Sulfoxides, especially DMSO, form coordination complexes with transition metals. The sulfoxide functional group occurs in several drugs (e.g. esomeprazole and armodafinil). Examples of sulfones in pharmacology include dapsone and promin.
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| (4-Ethylsulfonylphenyl)methanesulfonyl chloride | 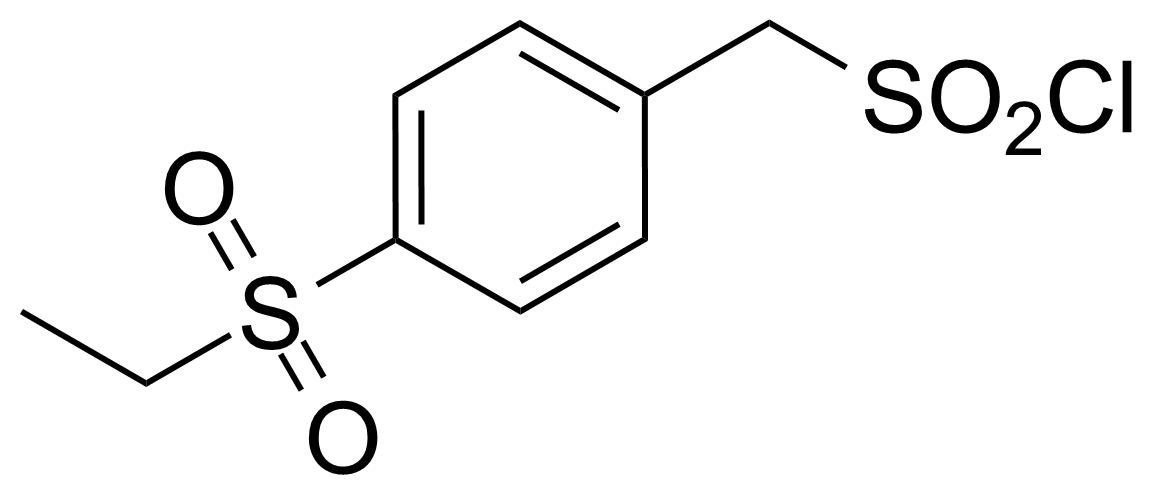 | [1542637-42-2] | GEO-03050 | |
| (4-Methylsulfonylphenyl)methanesulfonyl chloride | 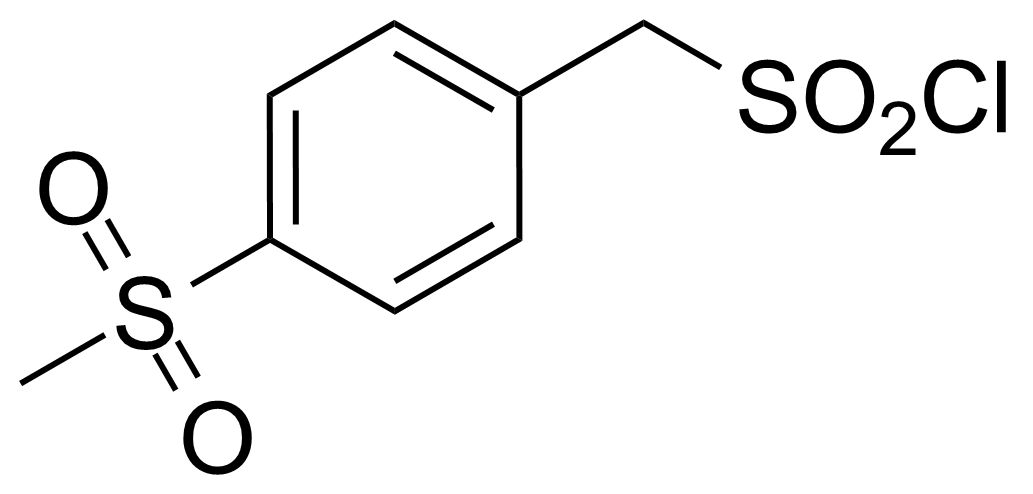 | [163295-77-0] | GEO-03041 | |
| Methyl p-tolyl sulfoxide | 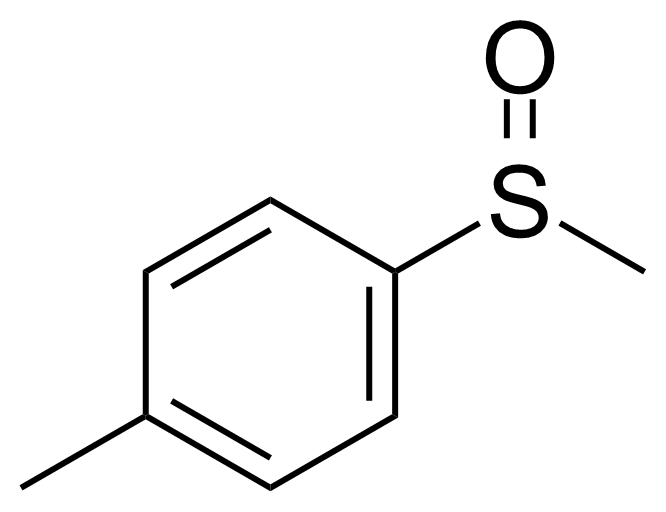 | [934-72-5] | GEO-04078 |