Hydrazines
Hydrazines are a class of chemical compounds with two nitrogen atoms linked via a covalent bond. They can be divided into few groups according to the type and degree of substitution (aliphatic, aromatic, symmetric, asymmetric). For the preparation of aliphatic hydrazines, the reaction of hydrazine with alkyl halides can be used, or by reduction of nitroso derivatives. Aromatic hydrazines can be prepared by reducing aromatic diazonium salts. Reduction of hydrazones and azines provides another approach to hydrazines. They are useful intermediates in synthesis, they can contribute in cyclocondensations to form heterocycles as pyrazoles and pyridazines which are a part of many bioactive commercial compounds. They act as nucleophiles in wide range of chemical reactions. They also find applications as dyes and in textile industry and photography.
| Product name | Structure | CAS# | G-code | |
|---|---|---|---|---|
| 3-Bromo-2-hydrazinyl-5-nitropyridine | 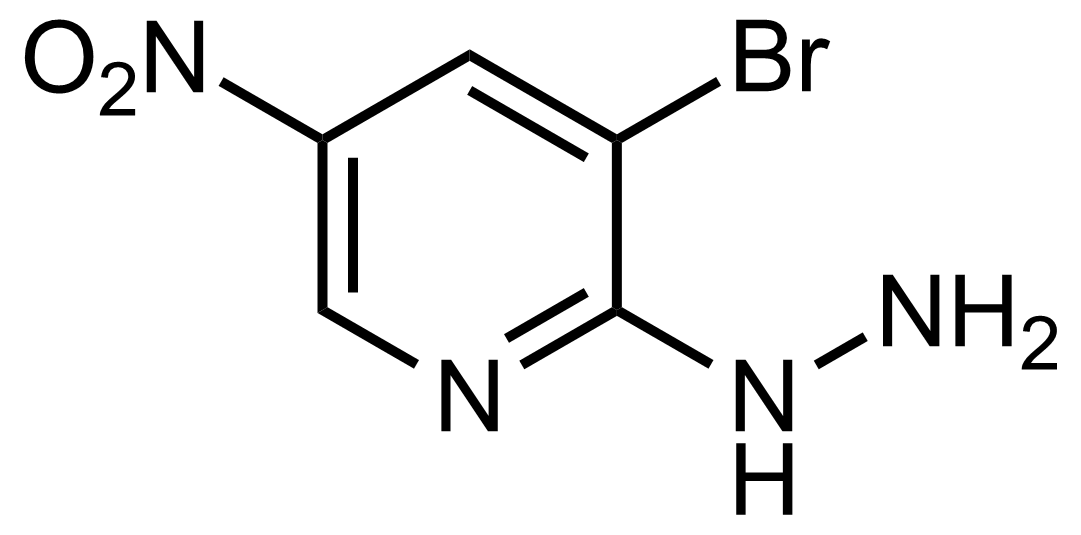 | [15862-38-1] | GEO-03654 | |
| 2-Chloro-3-hydrazinylpyrazine | 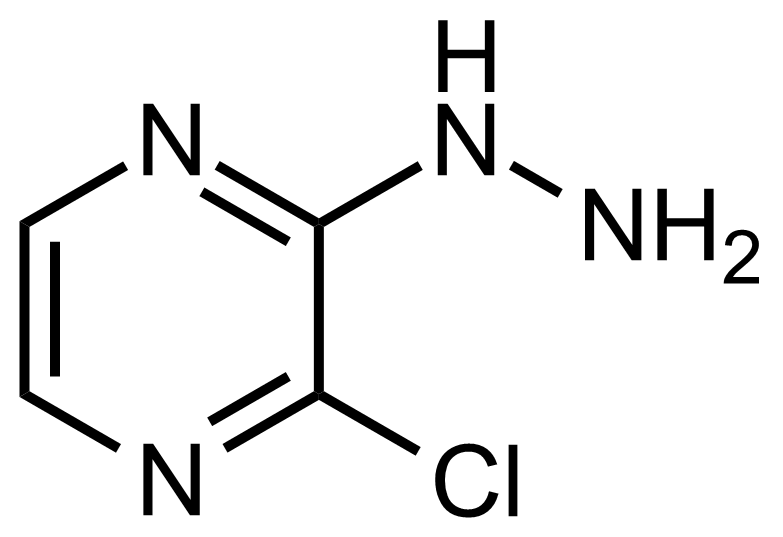 | [63286-28-2] | GEO-03685 | |
| 3-Chloro-p-tolylhydrazine hydrochloride | 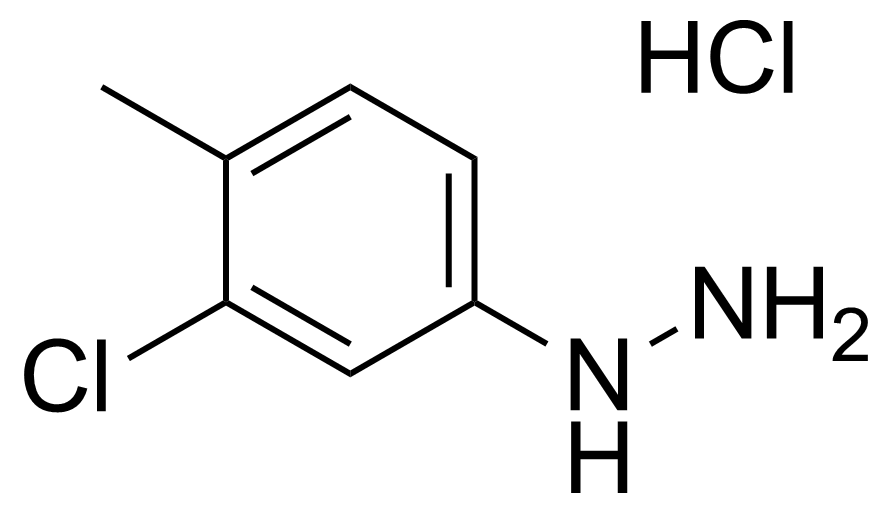 | [54812-56-5] | GEO-00811 | |
| 2-Ethoxy-4-fluoro-6-hydrazinylpyrimidine | 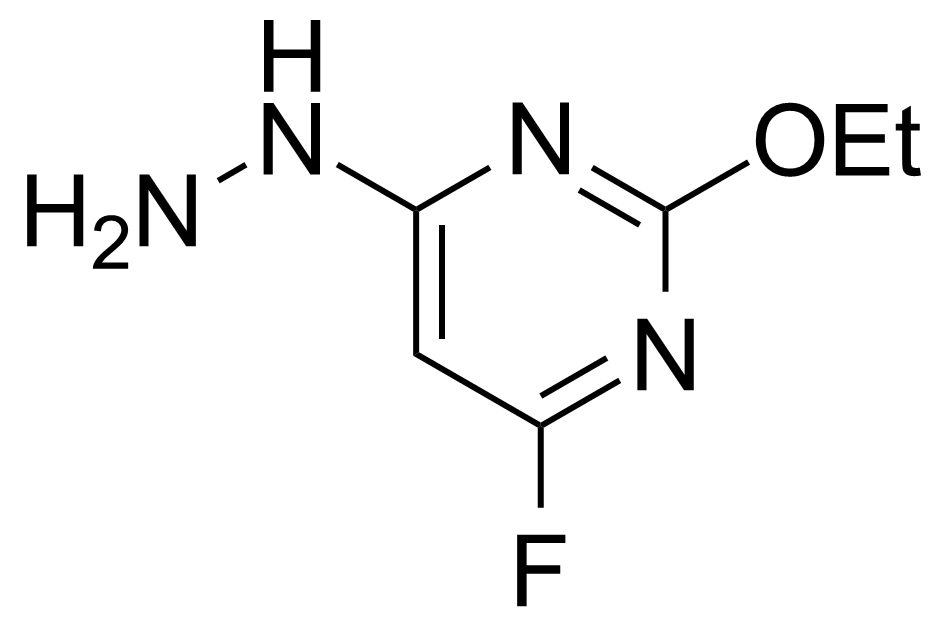 | [166524-66-9] | GEO-02650 | |
| 2-Hydroxyethylhydrazine | 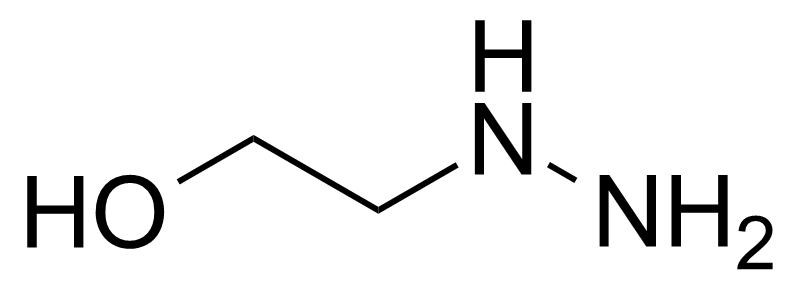 | [109-84-2] | GEO-01516 | |
| 4-Isopropylphenylhydrazine hydrochloride | 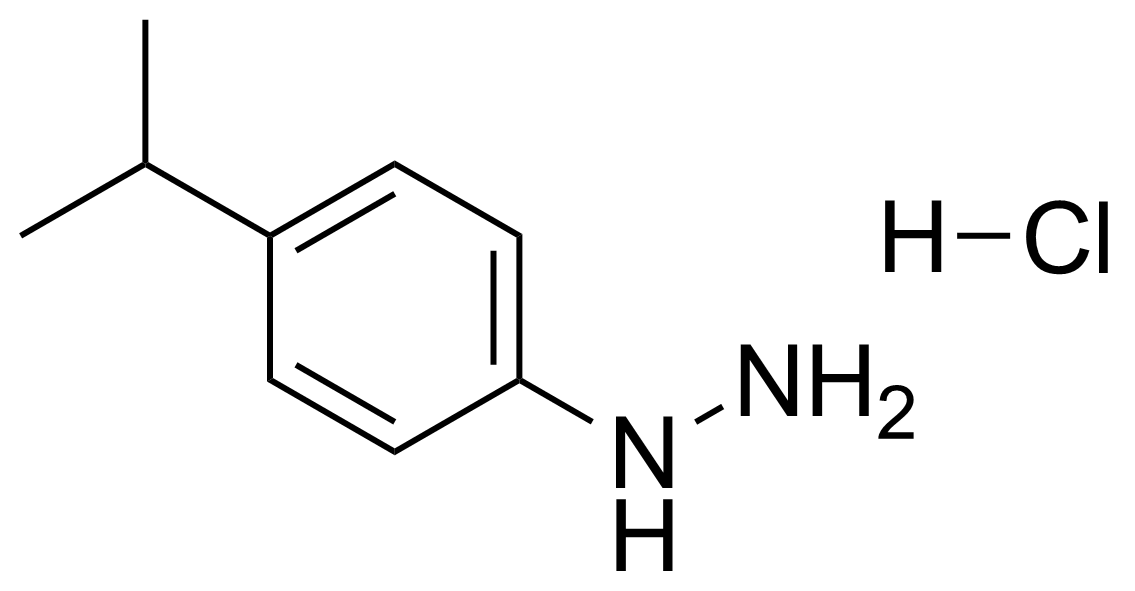 | [118427-29-5] | GEO-01623 |