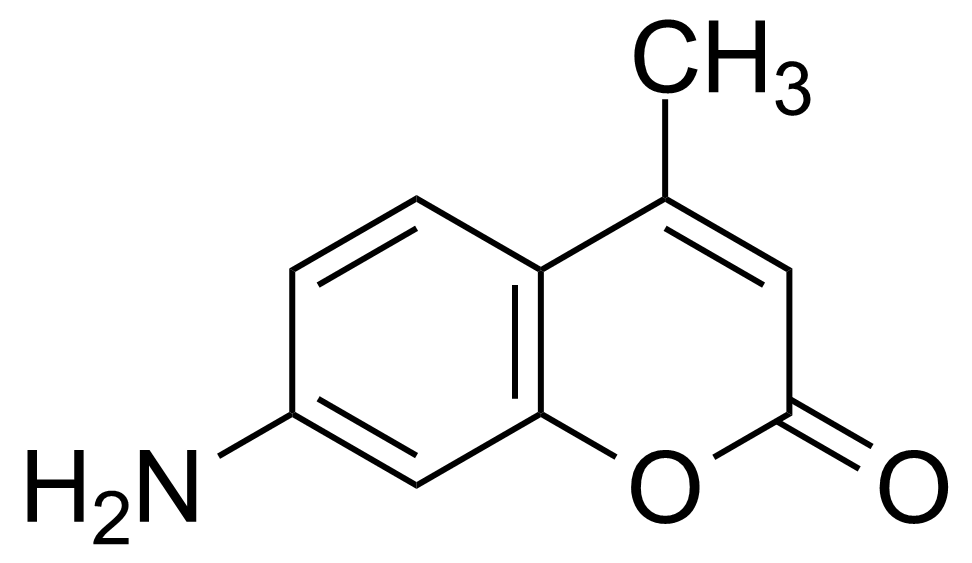
7-Amino-4-methylcoumarin
Produkt wurde eingestellt, aber wir haben Restbestände.
CAS-Nr.[26093-31-2]
G-CodeGEO-02697
SummenformelC10H9NO2
Molekulargewicht175.19
Synonyme
Coumarin 120 ; 7-Amino-4-methyl-2H-chromen-2-one
Für weitere Informationen oder eine Anfrage senden Sie uns bitte eine E-Mail oder nutzen Sie unser Kontaktformular
Regulatorische Informationen
Dieses Produkt ist nicht klassifiziert.
Produktkategorisierung
Hauptkategorie
Zweite Ebene
Dritte Ebene
Beschreibung
7-Amino-4-methylcoumarin ist eine nützliche chemische Verbindung mit vielfältigen Forschungsanwendungen. Wir freuen uns, qualitativ hochwertige 7-Amino-4-methylcoumarin in verschiedenen Größen (für Forschungs-, Pilotmaßstabs- oder Produktionsanwendungen) von Milligramm- bis Multi-Kilogramm-Chargen anbieten zu können, sodass Sie ganz einfach die richtige Menge für Ihre Bedürfnisse auswählen können.
Vollständige Beschreibung anzeigenUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of 7-Amino-4-methylcoumarin:
7-Amino-4-methylcoumarin [26093-31-2] (AMC), also known as Coumarin 120 belongs to the family of benzo-α-pyrones, called coumarins. It is a yellow crystalline solid with the melting point of 222-223 °C. It is soluble in common organic solvents.[1] 7-amino-4-methylcoumarin laser dye can emit laser in the range of 370-760 nm with the maximum λ at 354 nm (in ethanol). This compound is sensitive to the light and oxidizing agents therefore should be store in a dark, dry, tightly closed container. The most common method of preparation was published by Pechmann and Schwarz in 1899. It is based on the condensation of m-aminophenol with acetoacetic ester by heating in an alcohol in the presence of zinc chloride. Using this procedure, the AMC can be obtained in one step.[2]Application of 7-Amino-4-methylcoumarin:
Coumarin 120 is widely used as commercially available building block in the synthesis of fluorescent probes for a variety of sensing experiments in the enzymology. Such π-π conjugated system with electron-rich and charge transfer properties leads to the applications as fluorescent sensors for biological activities. Traditionally, coumarin substrates have been used to measure oxidative activities of cytochrome P450 (CYP) enzymes.[3] Moreover, it is widely used for peptide labeling in the study of proteases.[4] Coumarin-based chemosenors are used in selective detection of trace metals.[5] 7-Amino-4-methylcoumarin shows potential as antitubercular agent (the lowest MIC of 1 mg/L) by cell-wall-attacking mechanism of action.[6]Product categorization (Chemical groups):
Main category: Second level: Third level: ______________________________________________________________________________________[1] M. Zimmerman, E. Yurewicz, G. Patel Anal. Biochem. 1976, 70, 258. [2] H. Pechmann, O. Schwarz, Ber. Dtsch. Chem. Ges. 1899, 32, 3696. [3] D. Kim, Z. Wu, F. P. Guengerich J. Cell Biol. 2005, 280, 40319. [4] A. F. Kisselev, A. L. Goldberg Meth. Enzymol. 2005, 398, 364.
Kanaoka, T. Takahashi, H. Nakayama, T. Ueno, T. Sekine Chem. Pharm. Bull. 1982, 30, 1485.
Ishida, Y. Nakamura, T. Ohta, Y. Oe Molecules 2021, 26, 482. [5] L. Huang, W. Sheng, L. Wang, X. Meng, H. Duan, L. Chi RSC Adv, 2021, 11, 23597.
Muthusamy, K. Rajalakshmi, D. Zhu, W. Zhu, S. Wang, K. Lee, H. Xu, L. Zhao Sens. Actuators B Chem. 2021, 346, 130534. [6] R. Tandon, P. Ponnan, N. Aggarwal, R. Pathak, A. S. Baghel, G. Gupta, A. Arya, M. Nath, V. S. Parmar, H. G. Raj, A. K. Prasad, M. Bose, J. Antimicrob. Chemother. 2011, 66, 2543.
Ähnliche Produkte
| Produktname | Struktur | CAS-Nr. | G-Code | |
|---|---|---|---|---|
| Neu | 5-Acetyl-2-amino-4-(2-furanyl)-6-methyl-4H-pyran-3-carbonitrile | 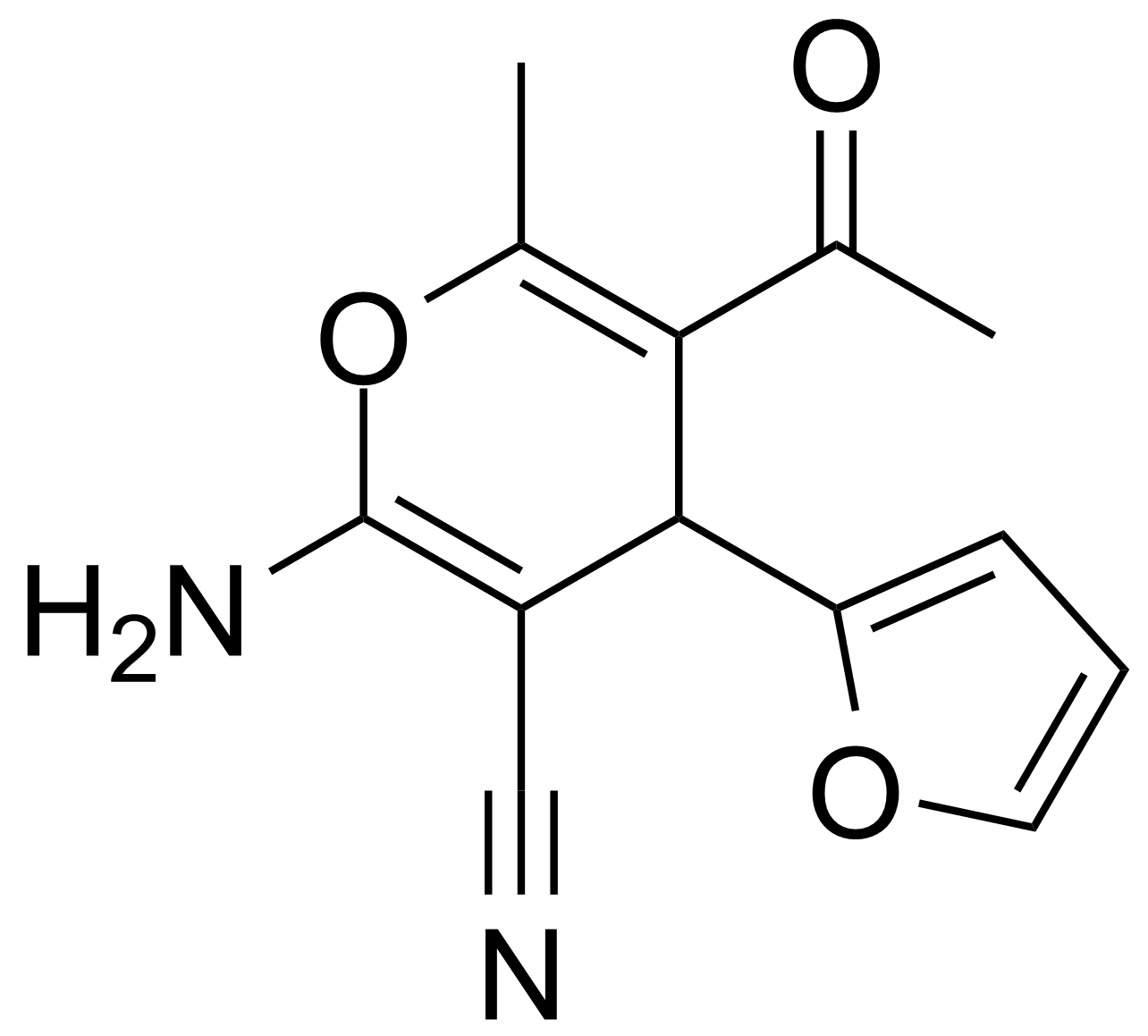 | [105263-08-9] | GEO-00016 |
| Neu | 5-Acetyl-2-amino-4-(4-methoxyphenyl)-6-methyl-4H-pyran-3-carbonitrile | 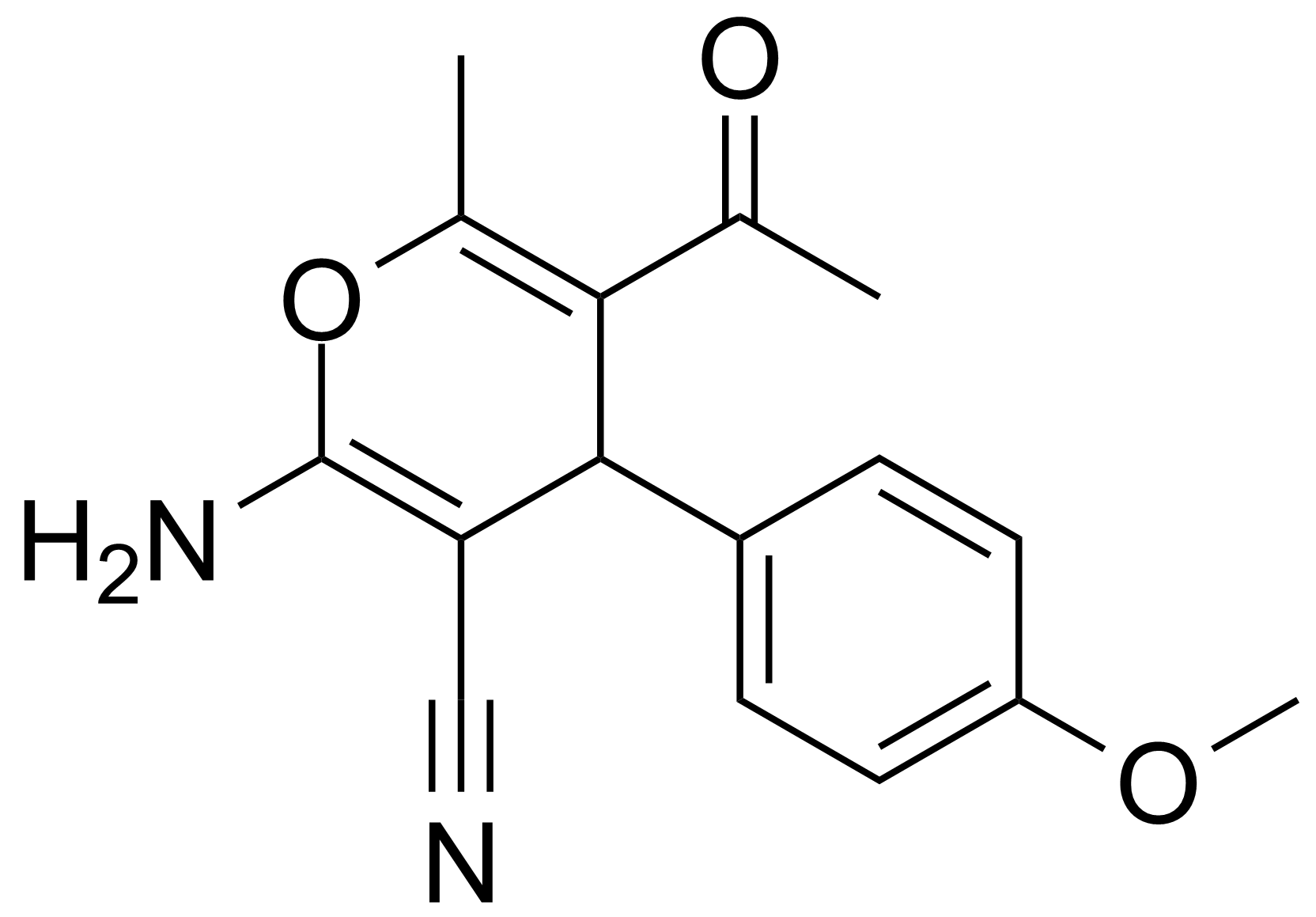 | [105263-07-8] | GEO-00017 |
| 5-Acetyl-2-amino-6-methyl-4-phenyl-4H-pyran-3-carbonitrile | 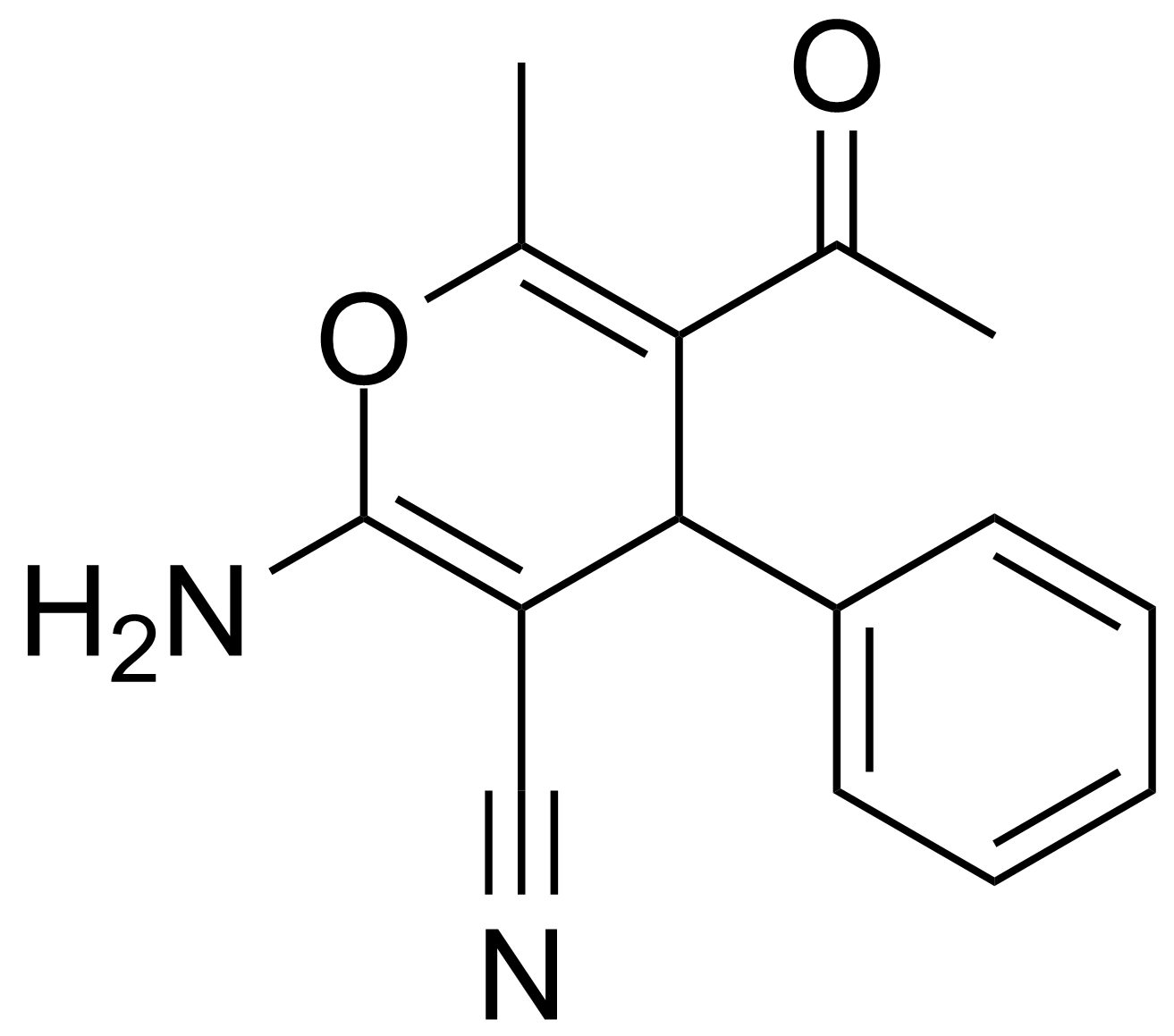 | [89809-89-2] | GEO-00018 | |
| Neu | 2-Acetylaminothiazole | 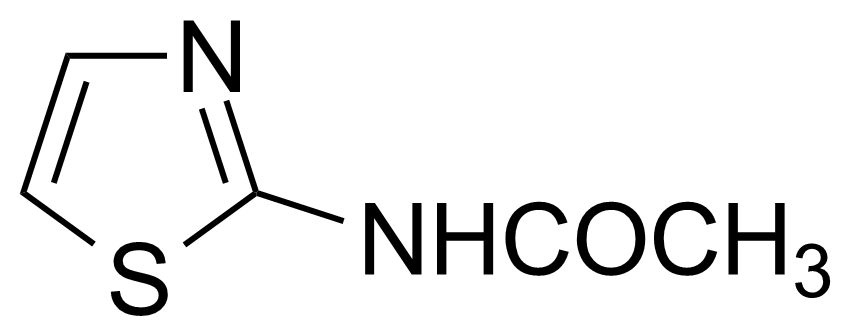 | [2719-23-5] | GEO-00020 |
| Neu | Allylcyclohexylamine | 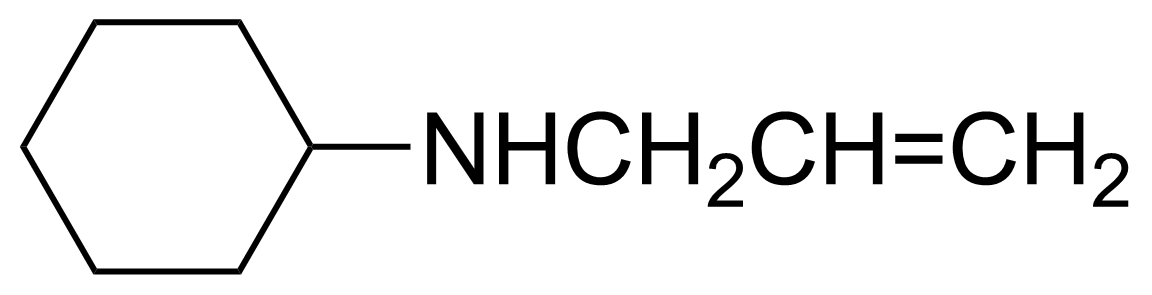 | [6628-00-8] | GEO-00059 |
| Neu | 4-Amino-2,1,3-benzothiadiazole | 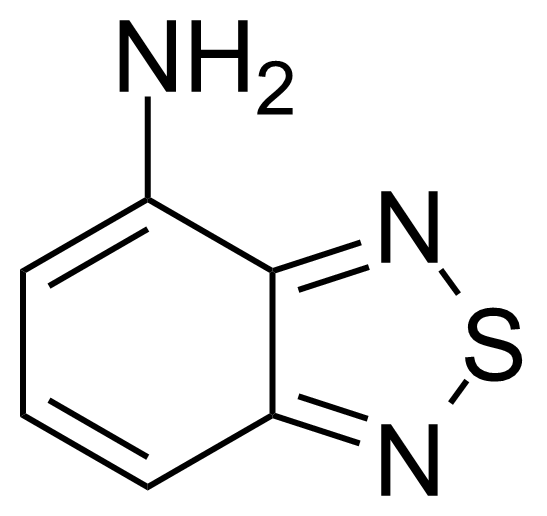 | [767-64-6] | GEO-00070 |
| Neu | 2-Aminobenzothiazole | 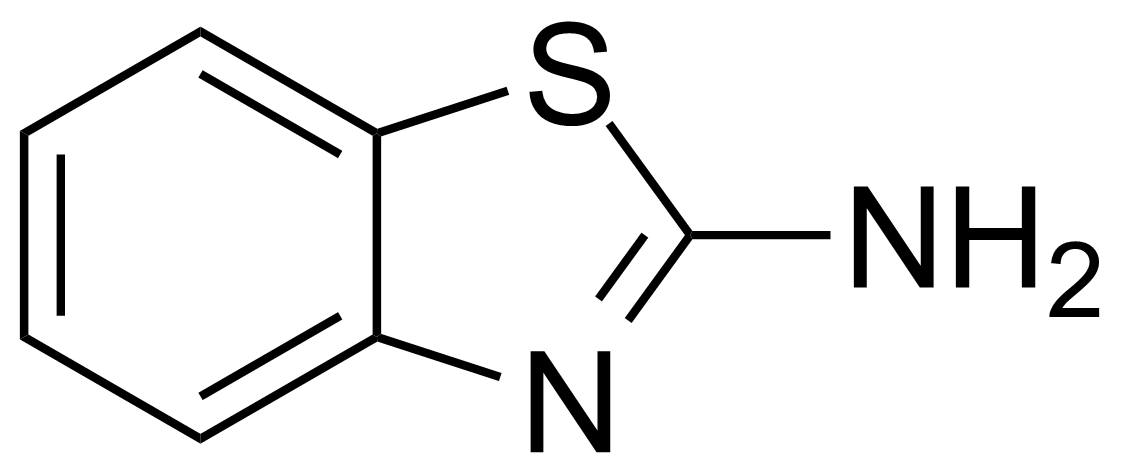 | [136-95-8] | GEO-00068 |
| Neu | 5-Amino-2-bromobenzoic acid | 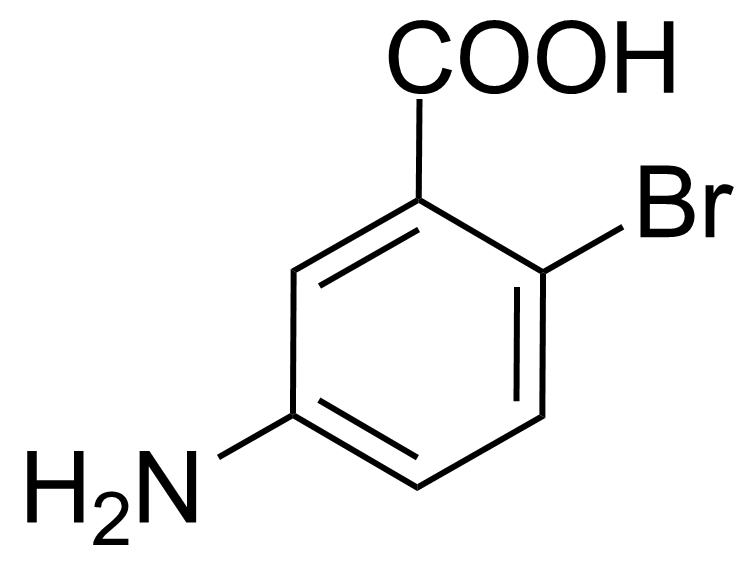 | [2840-02-0] | GEO-00082 |
| Neu | 2-Amino-6-bromobenzothiazole | 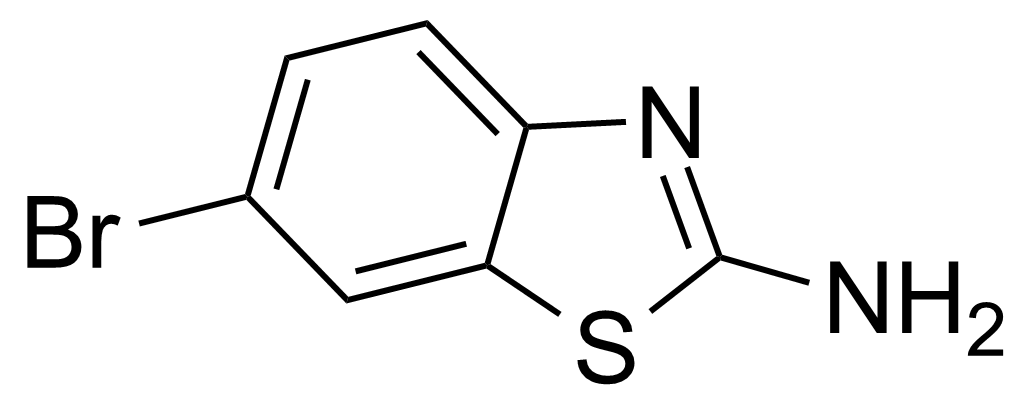 | [15864-32-1] | GEO-00083 |
| Neu | 6-Amino-5-bromo-1-methyluracil monohydrate | 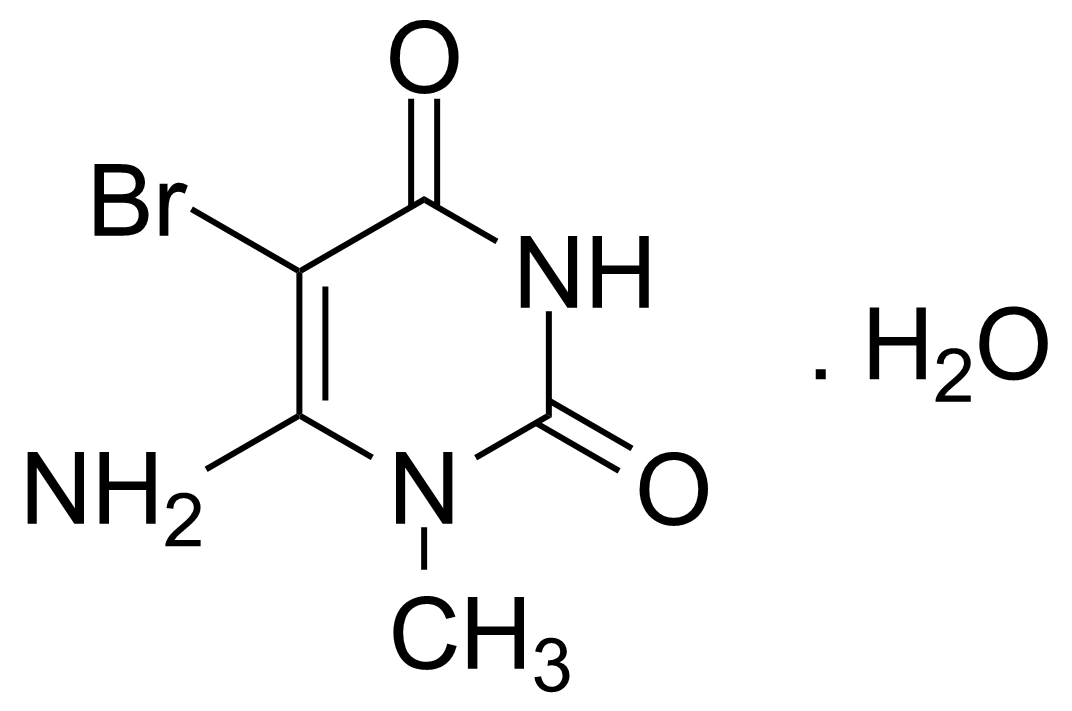 | [14094-37-2] | GEO-00087 |