 Januar 24, 2022
Januar 24, 2022D-Altrose – general description and application
Unfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of D-Altrose:
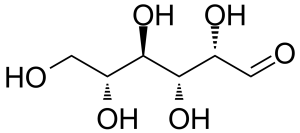
D-Altrose [1990-29-0] or (2S,3R,4R)-2,3,4,5,6-pentahydroxyhexanal is an aldohexose monosacharid, C-3 epimer of mannose. The pure compound is a colorless to offwhite crystalline solid with a melting point of 103-105 °C and a rotation at equilibrium [α] +32.6° in water.[1] It is an unnatural monosacharide that is soluble in water and almost insoluble in methanol.
D-Altrose can be prepared from levoglucosenone via D-altrosan, formed after stereoselective reduction and cis-hydroxylation.[2] D-altrose is formed as byproduct in the production of D-allose from D-psicose via epimerization catalysed with L-rhamnose isomerase.[3] Can be prepared from D-fructose using a combination of the immobilized D-tagatose 3-epimerase and D-arabinose isomerase but only with 6% overall yield.[4]
Application of D-Altrose:
D-altrose is used in synthesis of various unnatural analogues of biological active compounds like iminosugars.[5] Due to the lack of industrial production, application of D-altrose in different areas of research is still limited.
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
Regulatory Information:
- Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
______________________________________________________________________________________
[2] K. Matsumoto, T. Ebata, K. Koseki, H. Kawakami, H. Matsushita Bull. Chem. Soc. Jpn. 1991, 64, 2309.
[3] O. Pastinen, H. E. Schoemaker, M. Leisola Biochim. Biophys. Acta 2004, 1674, 68.
[4]B. T. Menavavu, W. Poonperm, K. Takeda, K. Morimoto, T. B. Granstrom, G. Takasa, K. Izumori J. Biosci. Bioeng. 2006, 102, 436.
[5]N. Araujo, S. F. Jenkinson, R. F. Martinez, A. F. G. Glawar, M. R. Wormald, T. D. Butters, S. Nakagawa, I. Adachi, A. Kato, A. Yoshihara, K. Akimitsu, K. Izumori, G. W. J. Fleet Org. Lett. 2012, 14, 4174.