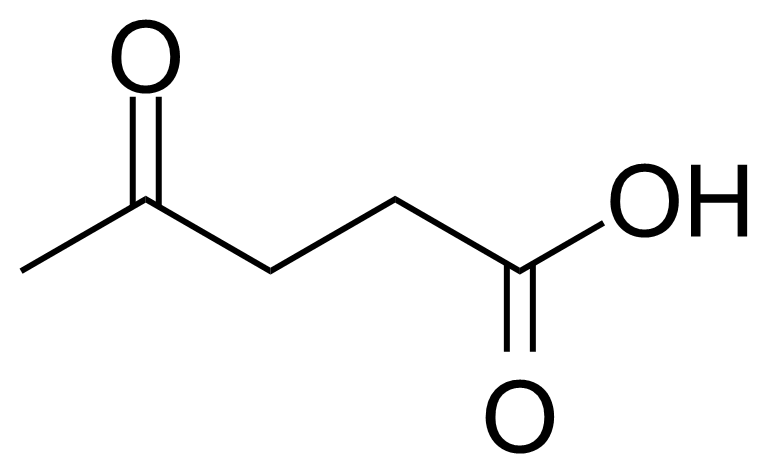
Levulinic acid
Numéro CAS[123-76-2]
G-codeGEO-04255
Numéro CE204-649-2
Formule moléculaireC5H8O3
Poids moléculaire116,12
Synonymes
4-Oxopentanoic acid ; 4-Oxovaleric acid
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires
Ce produit n’a pas été classé.
Catégorisation des produits
Catégorie principale
Deuxième niveau
Description
Levulinic acid est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des Levulinic acid de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description and preparation:
Levulinic acid, or 4-oxo-pentanoic acid, or b-acetylpropionic acid, or g-ketovaleric acid [123-76-2] is an organic acid belonging to keto acids group. In its pure form, it is a white crystalline solid with melting point of 33 °C.[1] It is soluble in water and polar organic solvents. Despite its low toxicity (LD50 1850 mg/Kg, oral, rat), as other acids, it can cause the acid burns and high concentrated solutions are irritating to the skin and mucous membranes.[2] The name, levulinic acid (originally levulinsäure, in german) was suggested by A. v. Grote and B. Tollens in 1874 as it was prepared from L-sugar (L-fructose) known as levulose.[3] Levulinic acid can be produced by acid hydrolysis of 5-hydroxymethylfufural (5-HMF), or by transformation of biomass/cellulose via formation of monomeric sugar derivatives.[4] In 1953 Quaker Oats developed a continuous process to produce levulinic acid from carbohydrate material such as starch, cellulose, or sugars.[5]Application of Levulinic acid:
It can be used as a raw material in organic synthesis, especially in the production process of some pharmaceuticals, in the preparation of 5-methyl-2-pyrrolidone,[6] g-valerolactone[7] and angelica lactone.[8] It has been recently found to be useful in the production of plasticizers and its esters as fragrance ingredients (fraistone) are used in the cosmetics production.[9] LA was identified as one of the twelve promising bio-based building blocks that can be subsequently converted to a number of high-value chemicals, fuels or materials.[10]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________
[1] S. M. Payne, F. M. Kerton Green Chem. 2010, 12, 1648. doi:10.1039/C0GC00205D
[2] V. Sunjic, J. Horvat, B. Klaic, S. Horvat, Kem. Ind. 1984, 33, 593.
[3] A. V. Grote, B. Tollens, Justus Liebigs Ann. Chem. 1875, 175, 181.
[4] B. F. McKenzie, Org. Synth. 1929, 9, 50. doi:10.15227/orgsyn.009.0050
[5] A. P. Dunlop, P. A. Wells Process for producing levulinic acid 1953, Quaker Oats Co, US2813900
[6] Y. Liu, Y. Wang, Y. Cheng, Z. Wei ChemistrySelect 2022, 7 (26), e202201191. doi:10.1002/slct.202201191
[7] S. K. Hussain, V. K. Velisoju, N. P. Rajan, B. P. Kumar, K. V. R. Chary ChemistrySelect 2018, 3 (22), 6186. doi:10.1002/slct.201800536
[8] C. G. S. Lima, J. L. Monteiro, T. M. Lima, M. W. Paixăo, A. G. Corrĕa ChemSusChem 2017, 11 (1), 25. doi:10.1002/cssc.201701469
[9] C. Antonetti, D. Licursi, S. Fulignati, G. Valentini, A. M. R. Galleti Catalysts 2016, 6 (12), 196. doi:10.3390/catal6120196
[10] S. H. Pyo, S. J. Glaser, N. Rehnberg, R. H. Kaul ACS Omega 2020, 5, 24, 14275. doi:10.1021/acsomega.9b04406
Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
| 5-Acetyl-2-amino-4-(2-furanyl)-6-methyl-4H-pyran-3-carbonitrile | 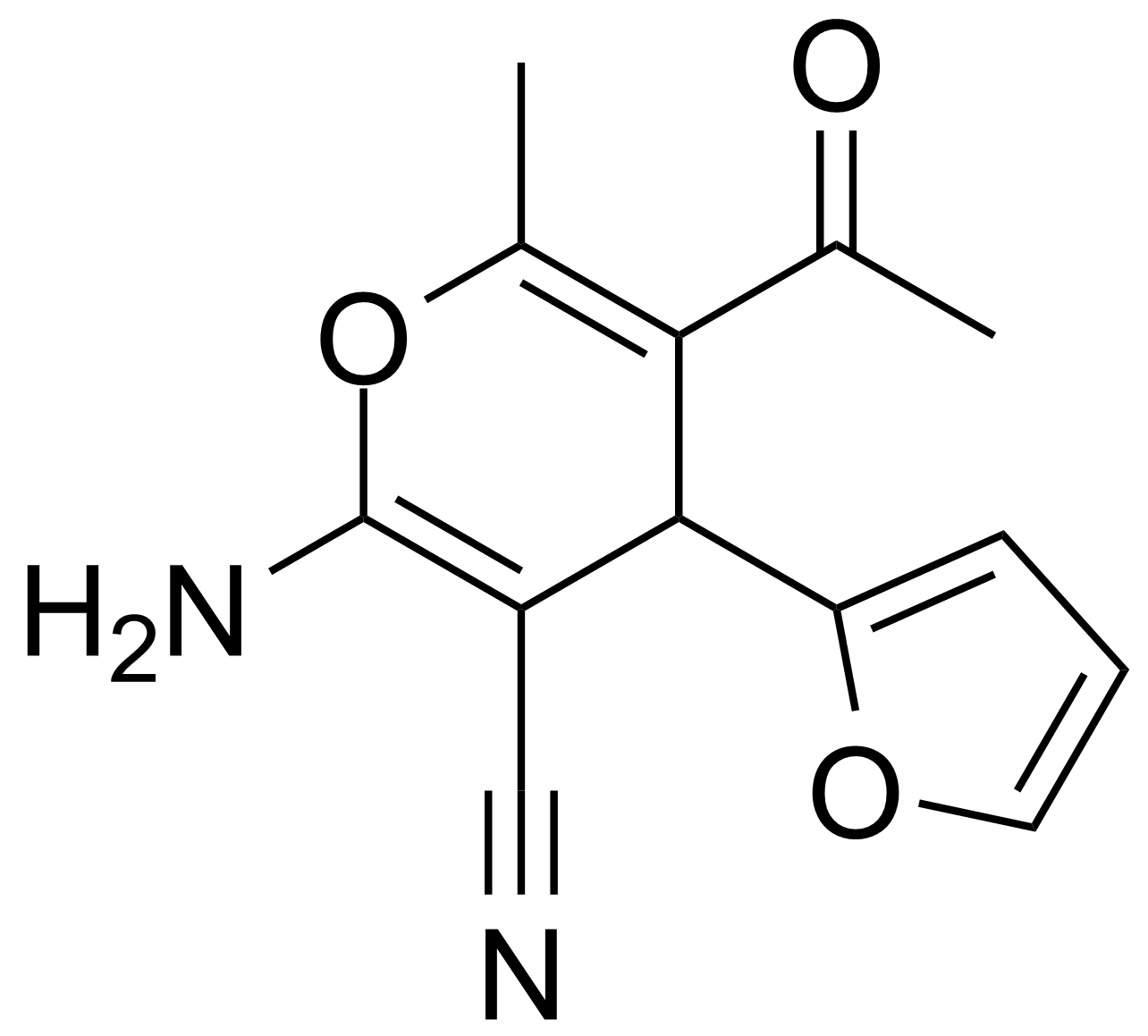 | [105263-08-9] | GEO-00016 | |
| 5-Acetyl-2-amino-4-(4-methoxyphenyl)-6-methyl-4H-pyran-3-carbonitrile | 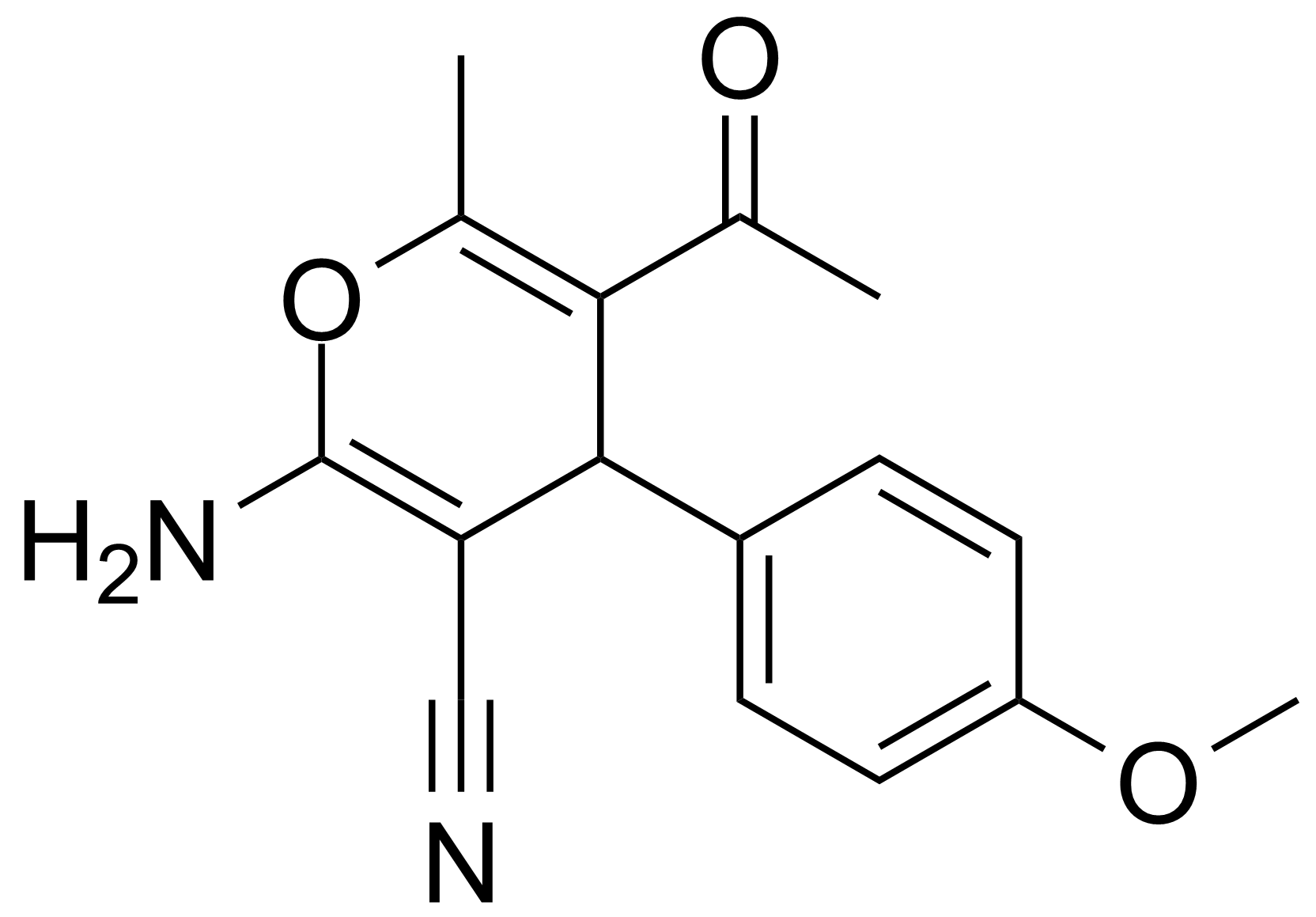 | [105263-07-8] | GEO-00017 | |
| 5-Acetyl-2-amino-6-methyl-4-phenyl-4H-pyran-3-carbonitrile | 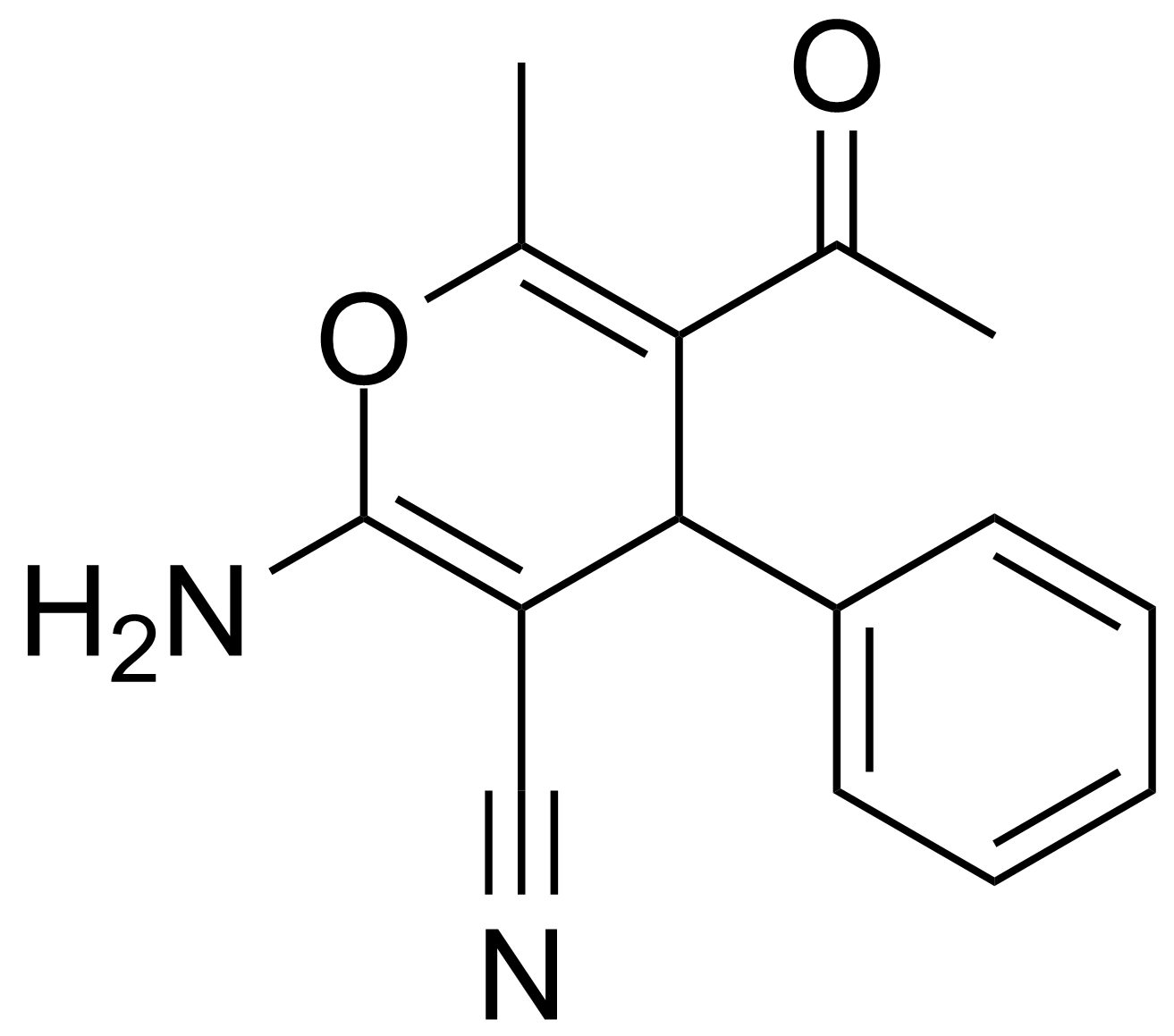 | [89809-89-2] | GEO-00018 | |
| 5-Acetyl-2,2′-bithienyl | 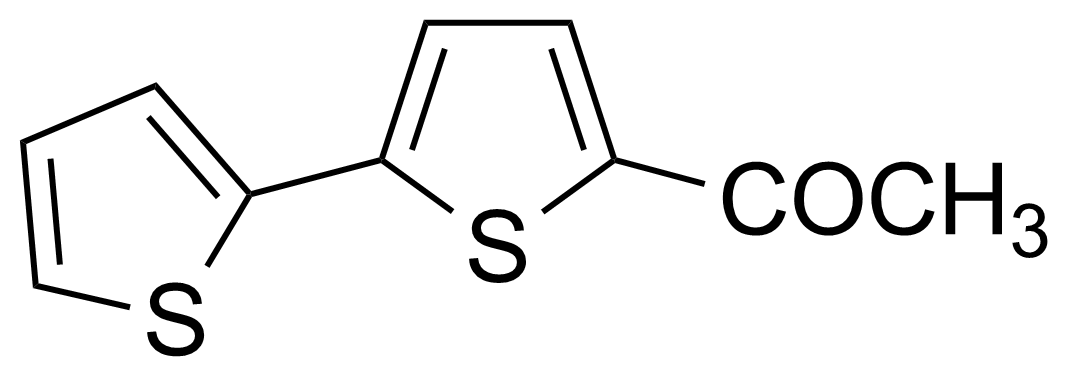 | [3515-18-2] | GEO-00022 | |
| 2-Acetyl-4-bromothiophene | 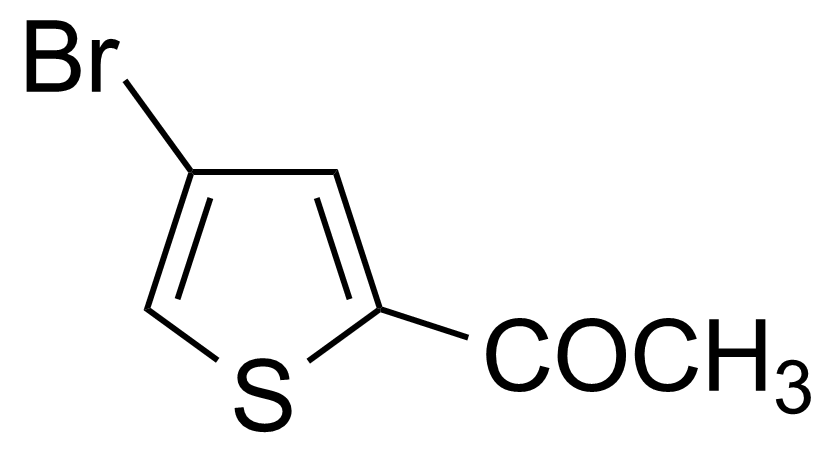 | [7209-11-2] | GEO-00023 | |
| Acetylcyclobutane | 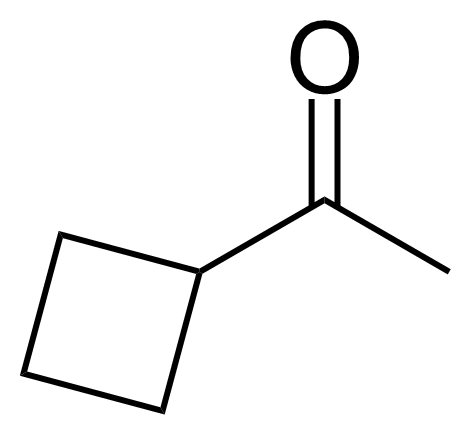 | [3019-25-8] | GEO-00006 | |
| 5-Acetyl-2,3-dihydrobenzo[b]furan | ![Structure of 5-Acetyl-2,3-dihydrobenzo[b]furan](https://georganics.sk/wp-content/uploads/2021/05/GEO-00030_5-Acetyl-23-dihydrobenzobfuran.png) | [90843-31-5] | GEO-00030 | |
| 4-Acetylimidazole | 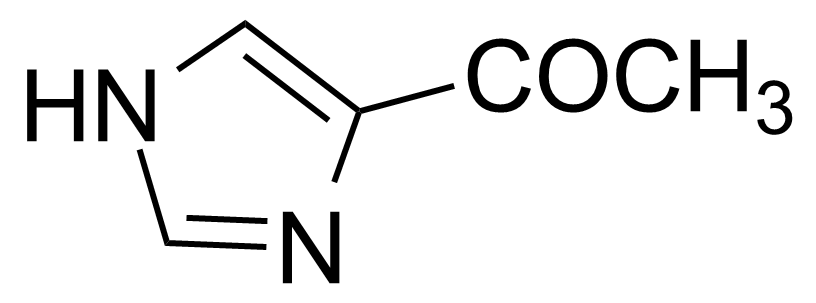 | [61985-25-9] | GEO-03036 | |
| 2-Acetyl-5-methylfuran | 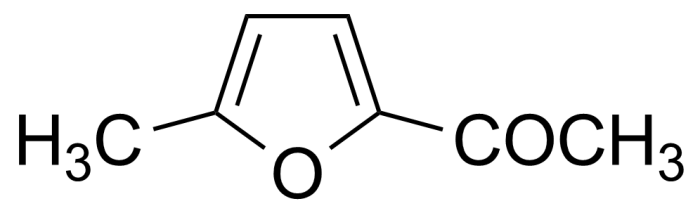 | [1193-79-9] | GEO-00034 | |
| 2-Acetyl-4-methyl-4-pentenoic acid methyl ester | 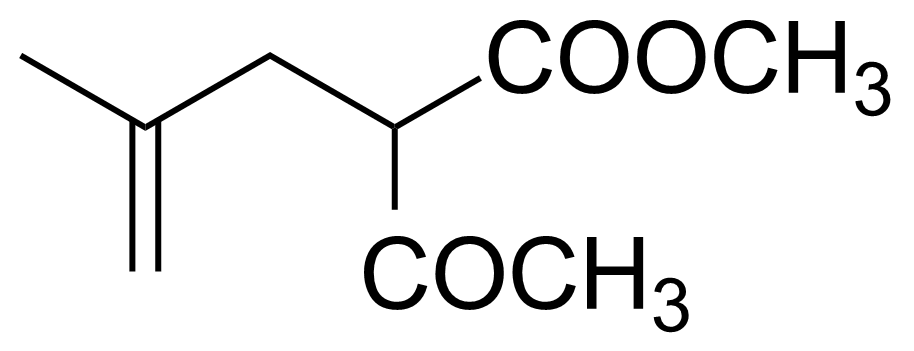 | [20962-71-4] | GEO-03240 |