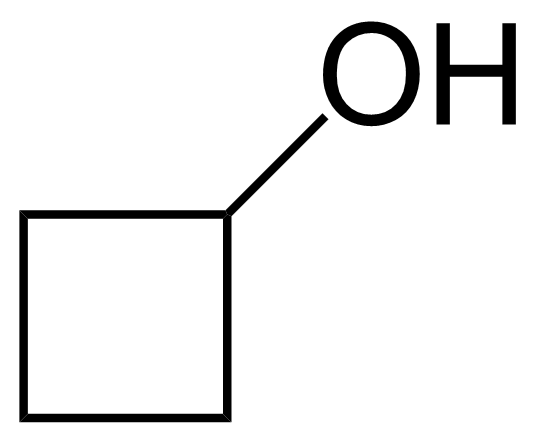
Cyclobutanol
Numéro CAS[2919-23-5]
G-codeGEO-03202
Numéro CE220-858-1
Formule moléculaireC4H8O
Poids moléculaire72,11
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires
Ce produit n’a pas été classé.
Catégorisation des produits
Catégorie principale
Deuxième niveau
Description
Cyclobutanol est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des Cyclobutanol de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of Cyclobutanol:
Cyclobutanol, or cyclobutyl alcohol or hydroxycyclobutane [2919-23-5] is an organic compound belonging to the class of alcohols. It is a colorless liquid with the boiling point of 122-124 °C.[1] Cyclobutanol and its vapors are highly flammable. It is only partially miscible in water, and readily soluble in common organic solvents. It can be prepared from cyclopropanemethanol by refluxing with aqueous hydrochlorid acid. Crude product is purified by distillation from 3-buten-1-ol as byproduct.[1] Another convenient option for the preparation is the reduction of cyclobutanone with lithium aluminium hydride.[2] Cyclobutanone can be prepared by the reaction of diazomethane with ketene in diethyl ether.[3]Application:
Cyclobutanol is a valuable reagent in synthetic chemistry. It was used in the preparation of tripeptide derivative as potent ACE inhibitor.[4] 1-Arylsubstituted cyclobutanols undergo silver-catalyzed ring opening forming the open-chain alkyl radical which can be either trapped by SelectFluor to furnish γ-fluorinated ketones.[5] The reaction without extrinsic radical scavenger gives the 1-tetralones via intramolecular cyclization.[6] Cyclobutanol ring expansion to 1-tetralones can be induced also electrochemically.[7] Alkenyl cyclobutanols can be used in catalyst-free selenylation/semipinacol rearrangement cascades to furnish β-selenylated cyclopentanones.[8]Product categorization (Chemical groups):
Main category: Second level: ______________________________________________________________________________________ [1] J. Salaün, A. Fadel Org. Synth. 1986, 64, 50. [2] J. D. Roberts, Ch. W. Sauer J. Am. Chem. Soc. 1949, 71, 3925. [3] P. Lipp, R. Köster Ber. Dtsch. Chem. Ges. 1931, 64, 2823. [4] T. Sawayama, M. Tsukamoto, T. Sasagawa, K. Nishimura, T. Defgughi, K. Takeyama, K. Hosoki Chem. Pharm. Bull. 1990, 38, 110. [5] H. Zhao, X. Fan, J. Yu, Ch. Z. J. Am. Chem. Soc. 2015, 137, 3490. [6] J. Yu, H. Zhao, S. Liang, X. Bao, Ch. Zhu, Org. Biomol. Chem. 2015, 13, 7924. [7] A. Petti, P. Natho, K. Lam, P. J. Parsons Eur. J. Org. Chem. 2021, 854. [8] D. Y. Kim Synth. Commun. 2019, 49, 2203.
Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
| Nouveau | 1-Acenaphthenol | 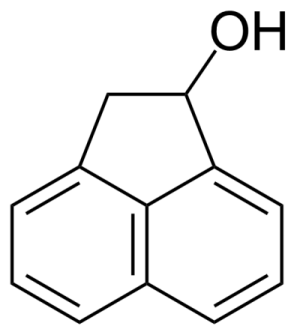 | [6306-07-6] | GEO-00001 |
| Nouveau | 7-Acetamido-4-hydroxy-naphthalene-2-sulfonic acid | 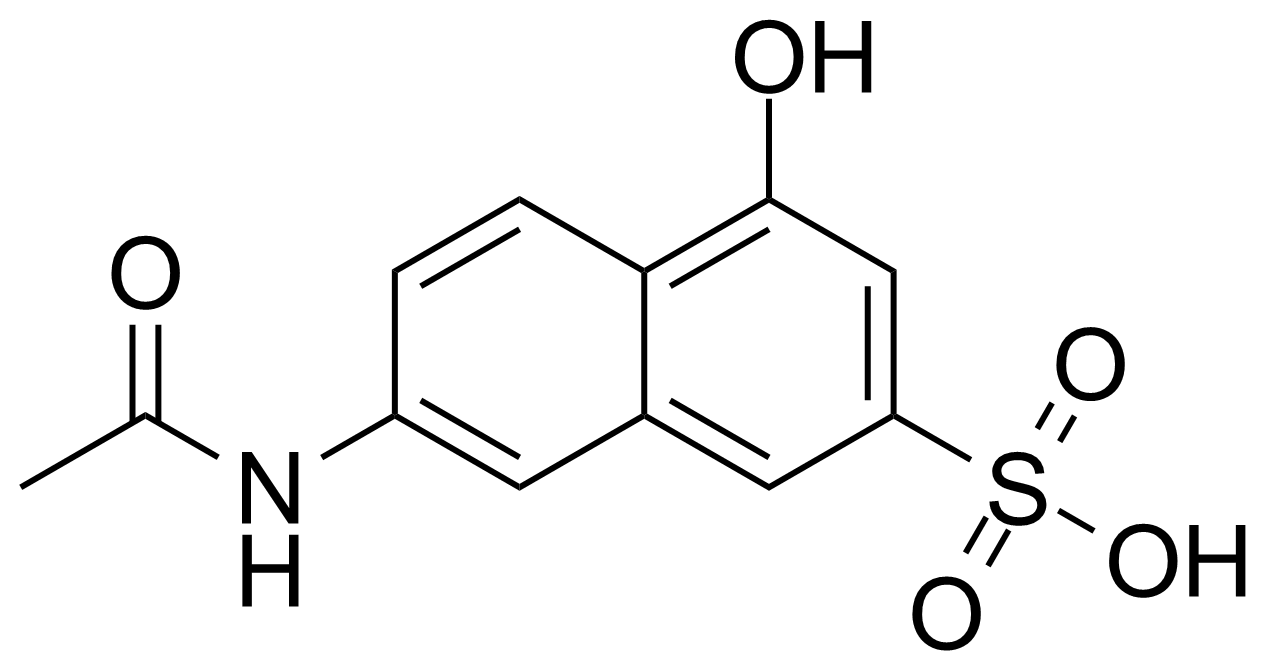 | [6334-97-0] | GEO-04013 |
| Nouveau | 1-Adamantanemethanol | 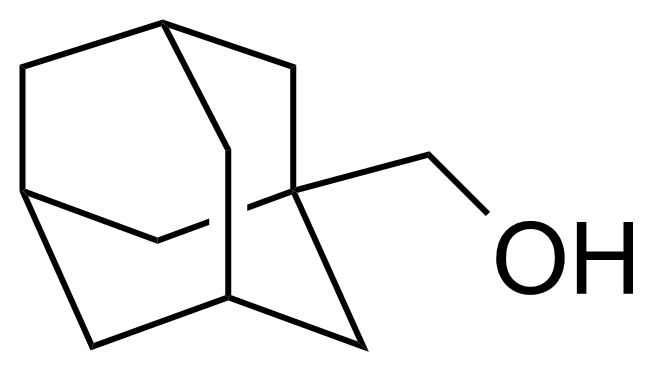 | [770-71-8] | GEO-04333 |
| beta-D-Allopyranose | 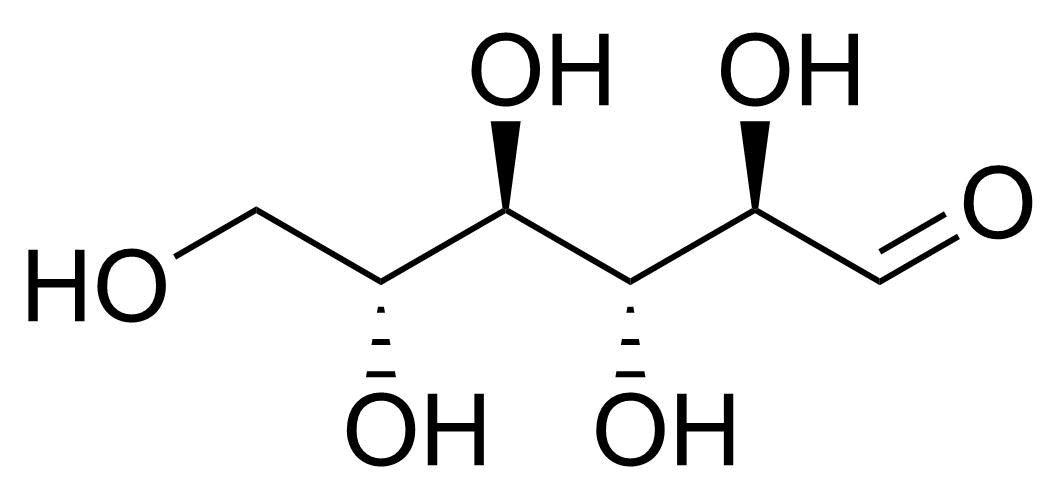 | [7283-09-2] | GEO-04660 | |
| Nouveau | D-Allose | 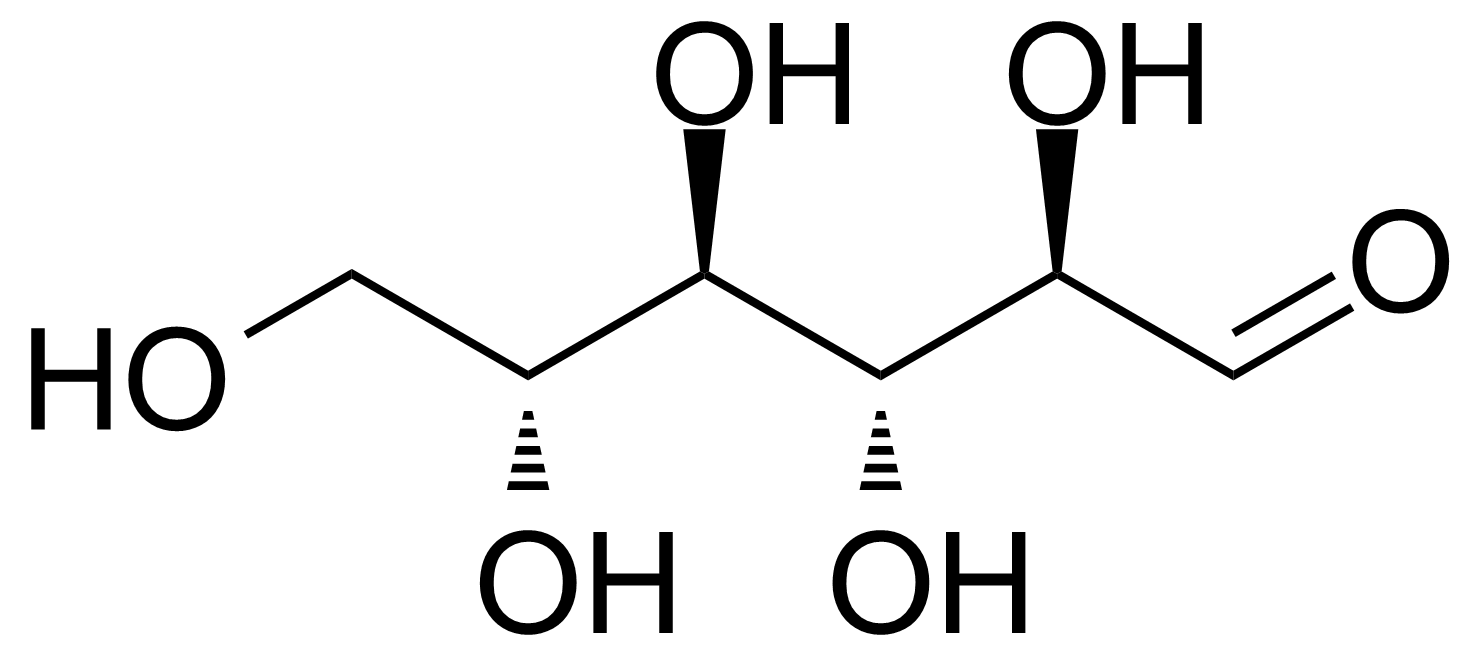 | [2595-97-3] | GEO-00057 |
| L-Allose | 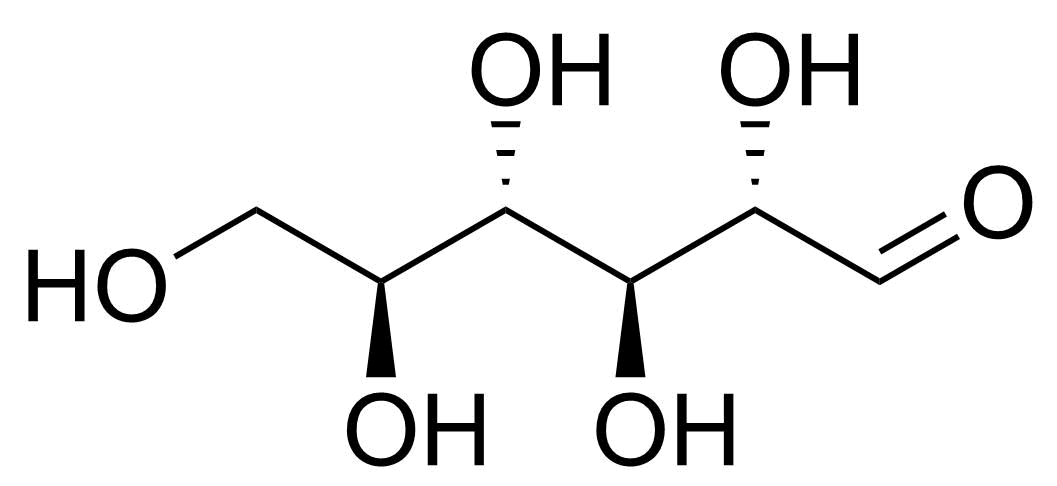 | [39392-62-6] | GEO-04661 | |
| Nouveau | 2-(Allyloxy)phenol | 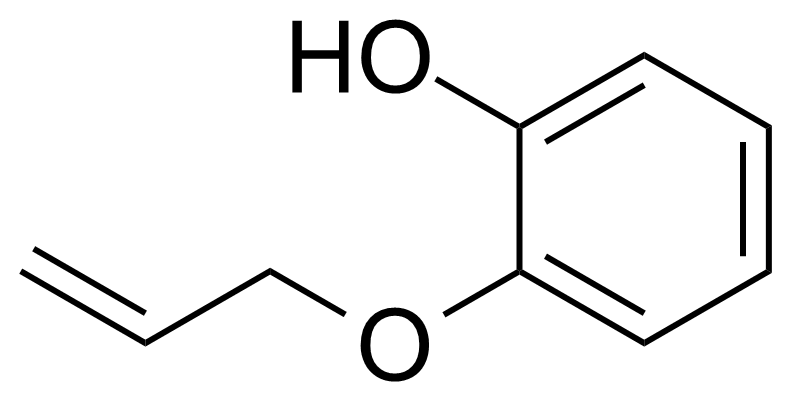 | [1126-20-1] | GEO-04471 |
| D-Altrose | 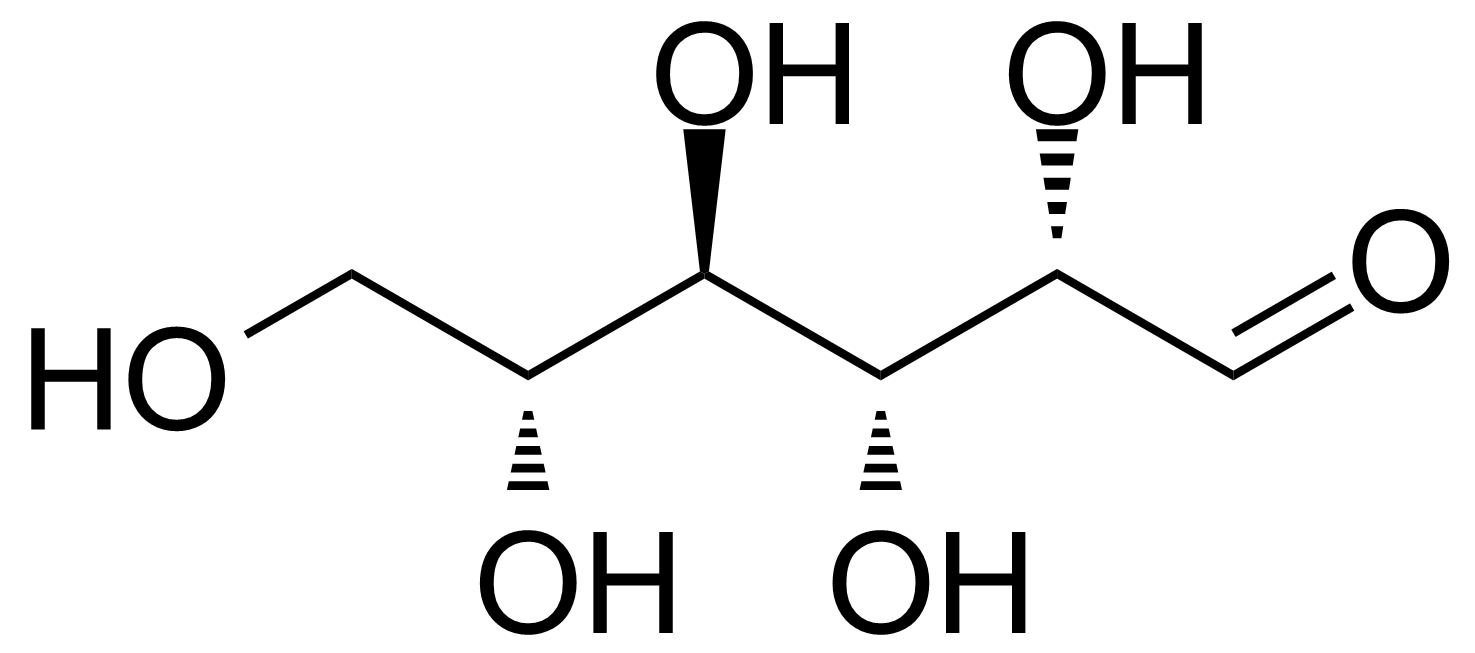 | [1990-29-0] | GEO-00058 | |
| L-Altrose | 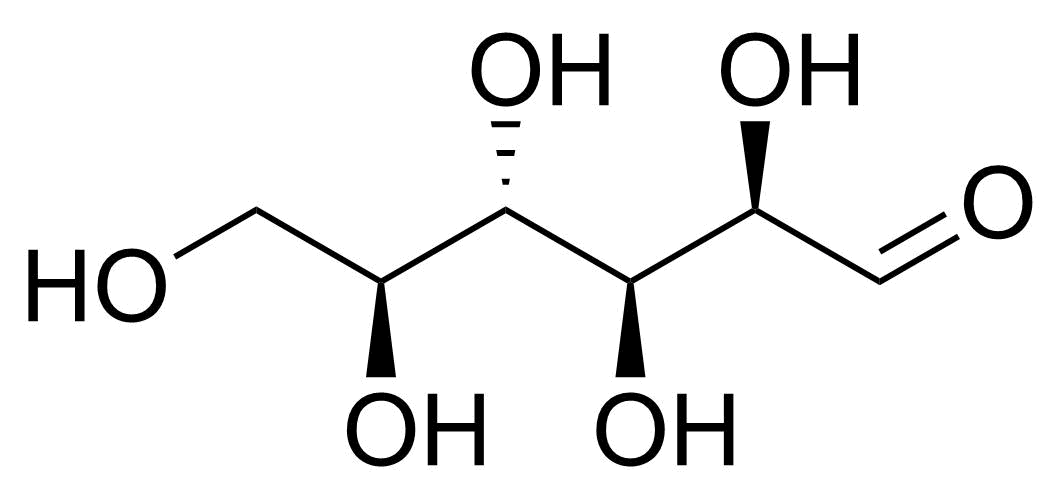 | [1949-88-8] | GEO-04662 | |
| Nouveau | (2R,4S)-2-Amino-4-cyclohexyl-4-hydroxybutanoic acid | 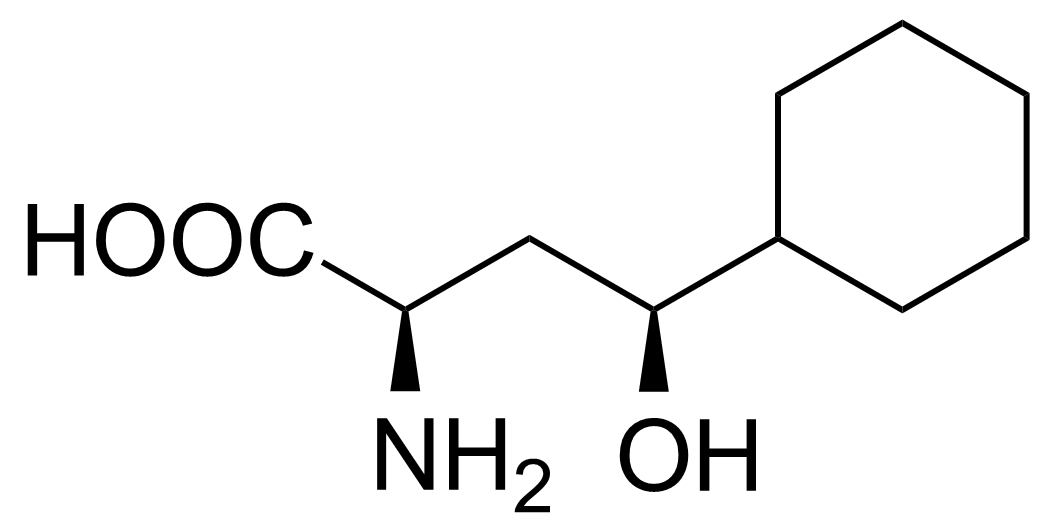 | [] | GEO-02717 |