 August 24, 2023
August 24, 2023L-pyroglutamic acid – description and application
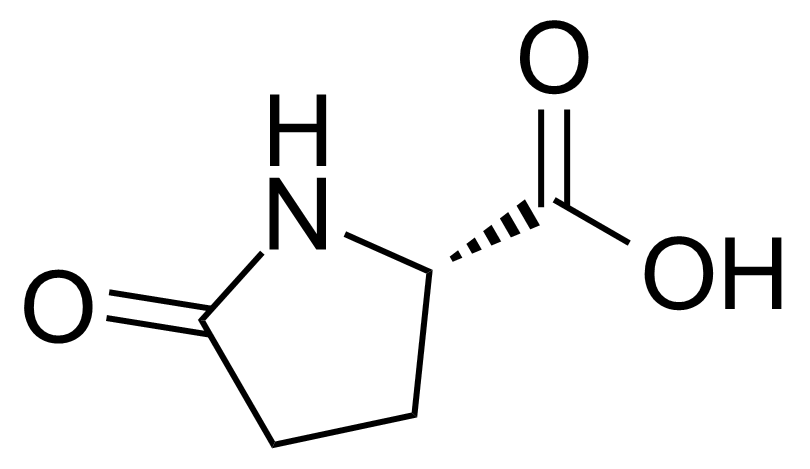
L-Pyroglutamic acid (also known as PCA, 5-oxoproline, pidolic acid) [98-79-3] is a five-membered lactam of glutamic acid. It is a pale yellow crystalline solid with the melting point of 155-157 °C.[1] It is a ubiquitous, but little studied natural amino acid derivative. It occurs naturally in fruits, some plant foods, dairy, and fermented products such as soy sauce. It is a metabolite in the glutathione cycle that is converted to glutamate by 5-oxoprolinase.[2]
Preparation of L-pyroglutamic acid:
PCA can be easily obtained by direct thermal dehydration of L-glutamic acid in water under reflux.[3]
Application of L-pyroglutamic acid:
In neurology, the brain-boosting effects of PCA were discovered in 1984. It plays an important role in the preservation and activity of the key neurotransmitters acetylcholine, gamma-aminobutyric acid (GABA) and glutamic acid.[4] It readily passes the blood-brain barrier to stimulate cognitive-enhancing function in rats[5] and reduce age-associated memory decline in humans.[6] In drug delivery, PCA esters can be used as dermal penetration enhancers for therapeutic agents having poor skin permeation or hair growth agents.[7] In chemical synthesis, it has been used as a versatile chiral building block in asymmetric synthesis of alkaloids, pharmaceuticals, and many other natural products.[8],[9] PCA is also known as a novel organic nonlinear optical (NLO) material for tunable UV harmonic generation down to 266 nm at room temperature.[10]
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
_______________________________________________________________________
[2] P. V. D. Werf, A. Meister Adv. Enzymol. Relat. Areas Mol. Biol. 1975, 43, 519. doi:10.1002/9780470122884.ch7
[3] P. M. Hardy Synthesis 1978, (4), 290. doi:10.1055/s-1978-24726
[4] T. Antonelli, V. Carlà, L. Lambertini, F. Moroni, C. Bianchi Pharmacol. Res. Commun. 1984, 16 (2), 189. doi:10.1016/S0031-6989(84)80094-6
[5] F. Drago, C. Valerio, V. D’Agata, C. Astuto, F. Spadaro, G. Continella, U. Scapagnini, Funct. Neurol. 1988, 3 (2), 137. pmid:3402813
[6] S. Grioli, C. Lomeo, M. C. Quattropani, G. Spignoli, C. Villardita Fundam. Clin. Pharmacol. 1990, 4 (2), 169. doi:10.1111/j.1472-8206.1990.tb00485.x
[7] W. T. Gibson, I. R. Scott Cosmetic composition comprising an ester of pyroglutamic acid 1992, Chesebrough Ponds USA Inc. US5158955.
[8] Y. Ohfune, M. Tomita J. Am. Chem. Soc. 1982, 104 (12), 3511. doi:10.1021/ja00376a048
[9] K. L. Yu, G. Rajakumar, L. K. Srivastava, R. K. Mishra, R. L. Johnson J. Med. Chem. 1988, 31 (7), 1430. doi:10.1021/jm00402a031
[10] M. Kitazawa, R. Higuchi, M. Takahashi Appl. Phys. Lett. 1994, 64, 2477. doi:10.1063/1.111602