 November 15, 2023
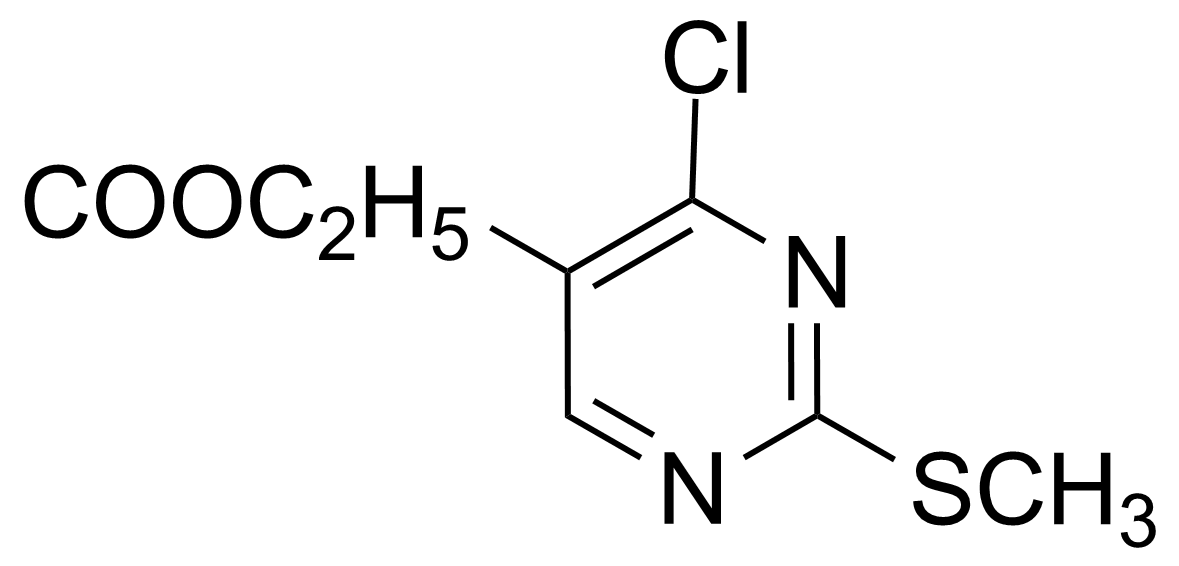
November 15, 2023Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate
Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate [5909-24-0] is white to yellow solid compound with the melting point of 58-60 °C.[1] It can cause skin, respiratory and serious eye irritation.
Preparation of Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate:
Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate can be prepared by a two step procedure. The condensation between S-methylisothiourea and diethyl ethoxymethylene malonate in basic conditions leads to the 4-oxopyrimidine sodium salt, which after treatment with phosphorous oxychloride under reflux affords desired 4-chloro derivative.[2]
Application:
Pyrimidines have been used as suitable starting materials for the synthesis of novel scaffolds that are parent to DNA bases and derivatives, thus targeting compounds with relevant biological and pharmacological properties such as anticancer, anxiolytic, antioxidant, antiviral, antifungal, anticonvulsant, antidepressant, and antibacterial activities.[3] Ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate was used in the synthesis of derivatives of pyrido[2,3-d]pyrimidin-7-one. These compounds are inhibitors of kinases such as Raf, including compounds that show anti-proliferative activity against cells, including against tumor cells, and are useful in the treatment of diseases including cancer.[4]
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
_______________________________________________________________________
[2] I. Grieco, M. Bissaro, D. B. Tiz, D. I. Perez, C. Perez, S. Redenti, E. Mariotto, R. Bortolozzi, G. Viola, G. Cozza, G. Spalluto, S. Moro, S. Federico Eur. J. Med. Chem. 2021, 216, 113331. doi:10.1016/j.ejmech.2021.113331
[3] S. M. Roopan, R. Sompalle Synth. Commun. 2016, 46, 645. doi:10.1080/00397911.2016.1165254
[4] A. Schoop, A. Backes, J. Vogt, L. Neuman, J. Eickhoff, S. Hannus, K. Hansen, P. Amon, I. Ivanov et al. Improved raf inhibitors 2009, GPC Biotech AG EP2112150A1