Pentachlorocyclopropane
1,1,2,2,3-PENTACHLORO-CYCLOPROPANE
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires

H315 – Provoque une irritation cutanée.
H319 – Provoque une sévère irritation des yeux.
H335 – Peut irriter les voies respiratoires.
P261 – Éviter de respirer les poussières/fumées/gaz/brouillards/vapeurs/aérosols.
P302+352 – EN CAS DE CONTACT AVEC LA PEAU: laver abondamment à l’eau et au savon.
P305+351+338 – EN CAS DE CONTACT AVEC LES YEUX: rincer avec précaution à l’eau pendant plusieurs minutes. Enlever les lentilles de contact si la victime en porte et si elles peuvent être facilement enlevées. Continuer à rincer.
P405 – Garder sous clef.
Catégorisation des produits
Description
Pentachlorocyclopropane est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des Pentachlorocyclopropane de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteDescription of Pentachlorocyclopropane:
Pentachlorocyclopropane (PCCP) [6262-51-7] is a colorless mobile liquid with a faint minty odour and a boiling point of 70 °C/19 mmHg.[1] The chemical stability of pentachlorocyclopropane is due to its intramolecular hydrogen bonds. This allows it to be stored at room temperature without undergoing any reactions or decomposing. It is thermally unstable, undergoing rapid ring-opening isomerization above 100 °C to give exclusively 1,1,3,3,3-pentachloropropene.[2] Causes tremor and necrotic gastrointestinal changes in dermal lethal-dose studies of rats (2 g/kg). Causes visual field changes, somnolence, and convulsions in oral lethal-dose studies of rats (78 mg/kg).[3]Application:
Triaminocyclopropenium:
Triaminocyclopropenium (TAC) salts were first reported in 1971 by Yoshida and Tawara[4] and have attracted much interest since that time. Despite the steric strain of the three-membered ring, these cations are remarkably unreactive. They are stable in boiling water, and it was reported that the NTf2- salts, which are ionic liquids, can have thermal decomposition onset temperatures above 400°C. TAC cations can be readily prepared in one step from a secondary amine and either tetrachlorocyclopropene or pentachlorocyclopropane. Generally, the cyclopropane is used as it saves one step (conversion of the cyclopropane to the cyclopropene) in the synthesis, even though a larger amount of amine is required to remove the HCl generated during the reaction.[5] It was used in synthesis of tris(dialkylamino)-cyclopropenium (TDAC) bistriflamide ionic liquids.[6] The use of tris(amino)cyclopropenium (TAC) ions as a novel class of phase-transfer catalysts was reported for the reaction of epoxides with carbon dioxide. TACs can be oxidized to form stable and even isolable radical dications at a relatively low oxidation potential (∼1.2 V vs standard calomel electrode SCE), a fact that has been known since the early 1970s from work by Weiss[7] and Yoshida.[8] Recently, Sanford investigated TACs for use in organic flow batteries due to their robustness toward redox cycling.[9] They observed photoreduction of TAC dication to TAC by DME solvent upon exposure to ambient light thus TAC can serve as electrophotocatalyst. The radical dication undergoes photoexcitation with visible light to produce an excited-state species with oxidizing power (3.33V vs. SCE) sufficient to oxidize benzene and halogenated benzenes via single-electron transfer (SET), resulting in C-H/N-H coupling with azoles.[10]Pentachlorocyclopropane:
Pentachlorocyclopropane is used in preparation of a sulfur[11] and nitrogen[12] heterocyclic ring substituted cyclopropane compounds with the application in an organic light-emitting devices. Such compounds have excellent hole transport property and stability. When the organic electroluminescent material is utilized as a doping material, a blocking material, a charge injection material, or similar roles in an organic semiconductor, particularly in an organic electronic device, it enables the organic electroluminescent material to operate at low voltage. This, in turn, enhances the electroluminescent efficiency and extends the device's service life. Very recently the first deltic ionic liquid crystals based on aminocyclopropenium ions have been developed.[13]Product categorization (Chemical groups):
Main category: Second level: _______________________________________________________________________Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
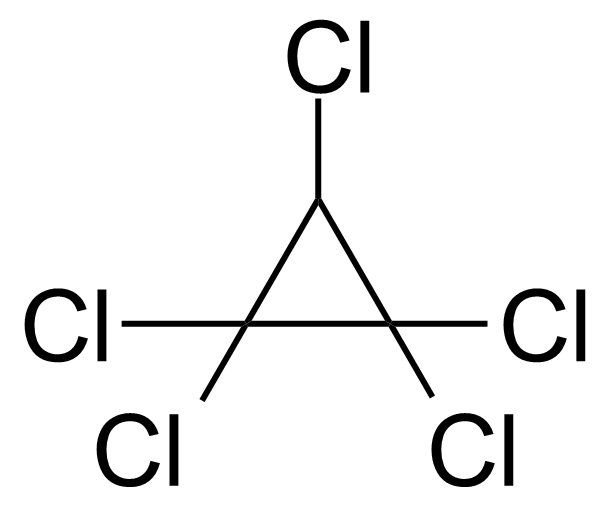
| Nouveau | 2,6-Dichloro-N-cyclohexylnicotinamide | 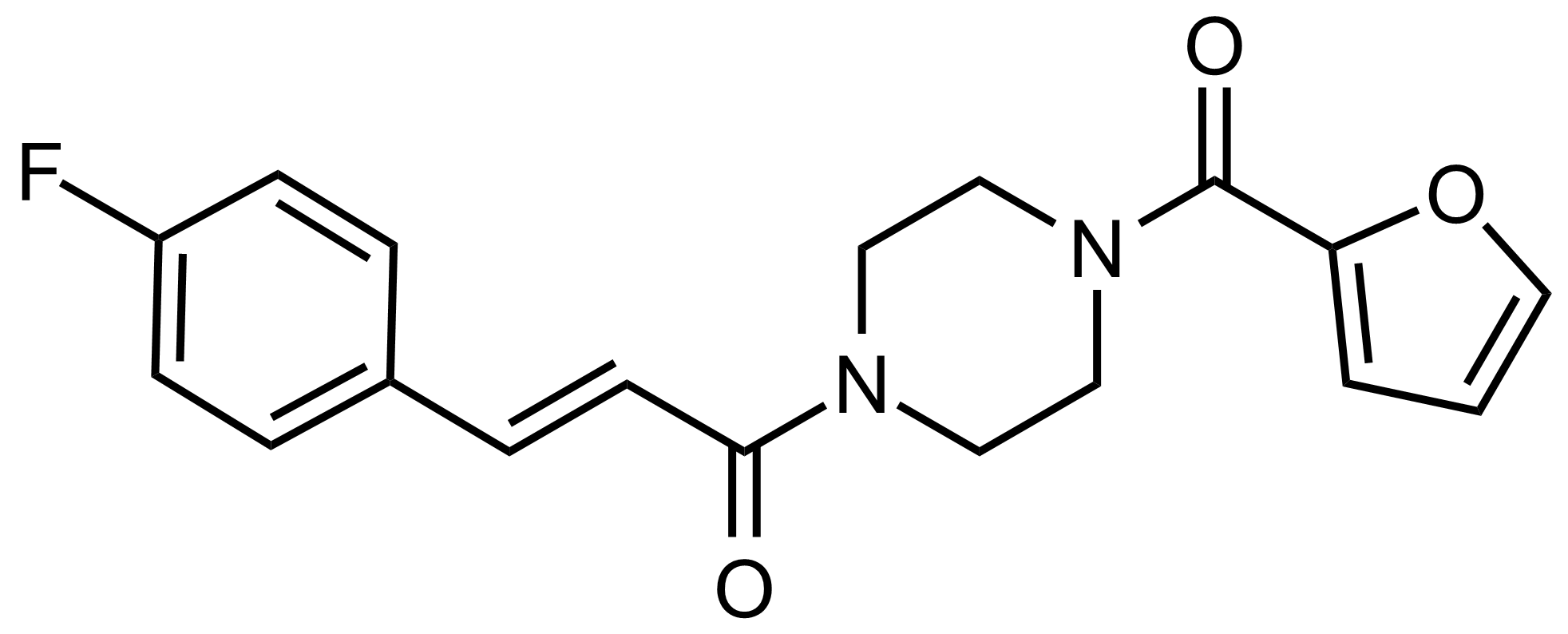 | N/A | GEO-03562 |
| Nouveau | 1-Bromo-3,3-dimethylbutane | 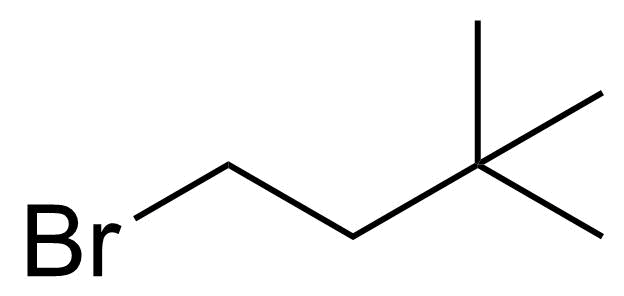 | [1647-23-0] | GEO-04482 |
| Nouveau | 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-alpha-D-glucopyranosyl chloride | 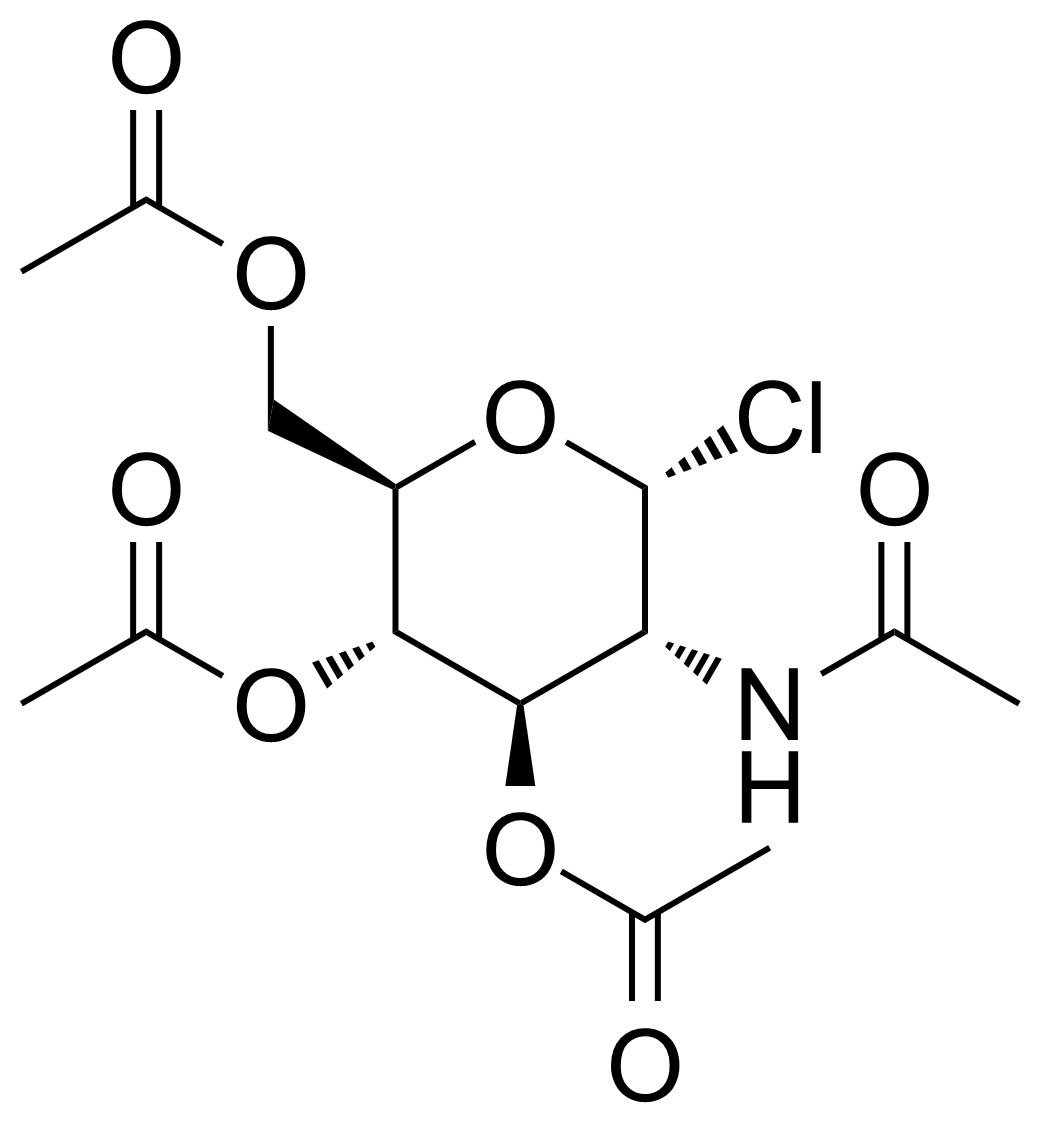 | [3068-34-6] | GEO-03374 |
| Nouveau | Acetochloro-beta-D-glucose | 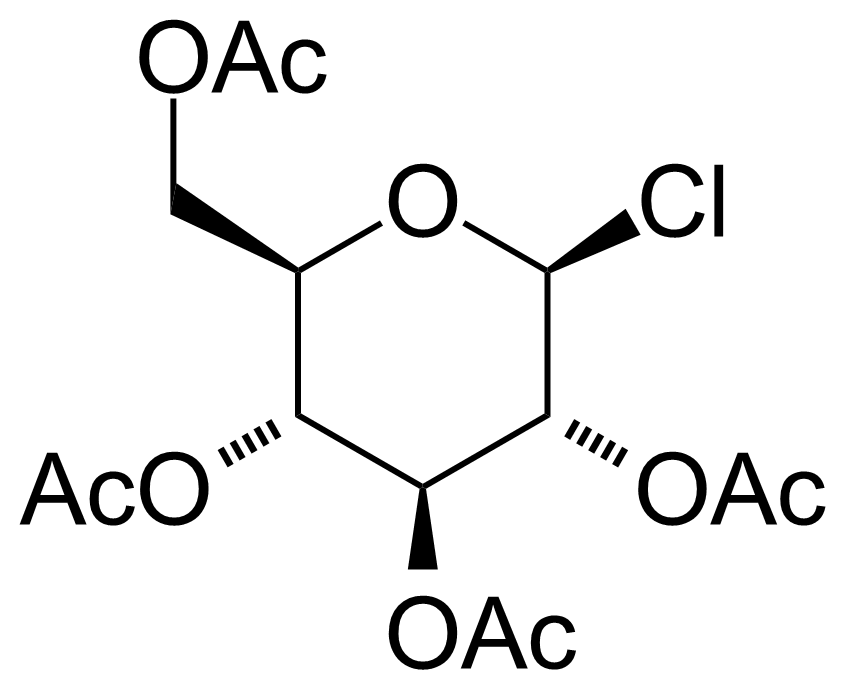 | [4451-36-9] | GEO-00008 |
| Nouveau | 2-Acetyl-4-bromothiophene | 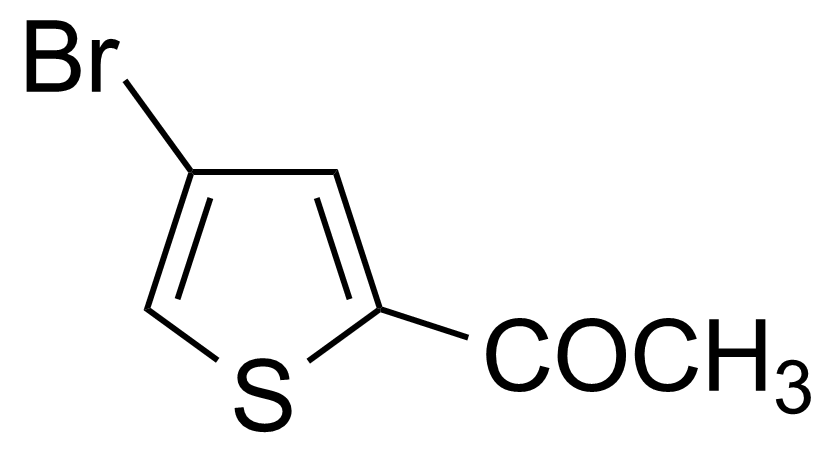 | [7209-11-2] | GEO-00023 |
| Nouveau | 5-Amino-2-bromobenzoic acid | 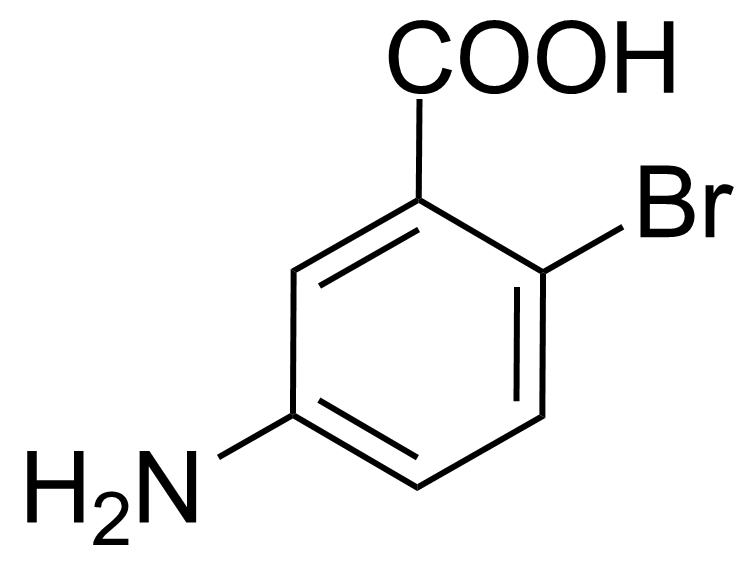 | [2840-02-0] | GEO-00082 |
| Nouveau | 2-Amino-6-bromobenzothiazole | 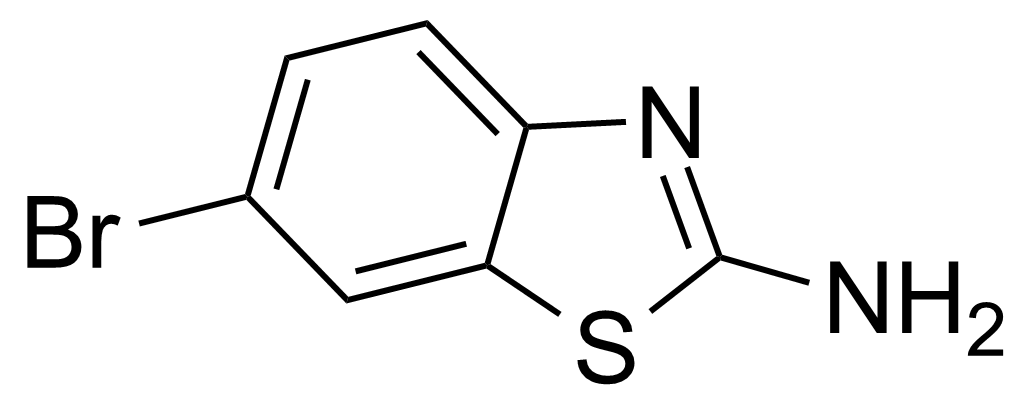 | [15864-32-1] | GEO-00083 |
| Nouveau | 6-Amino-5-bromo-1-methyluracil monohydrate | 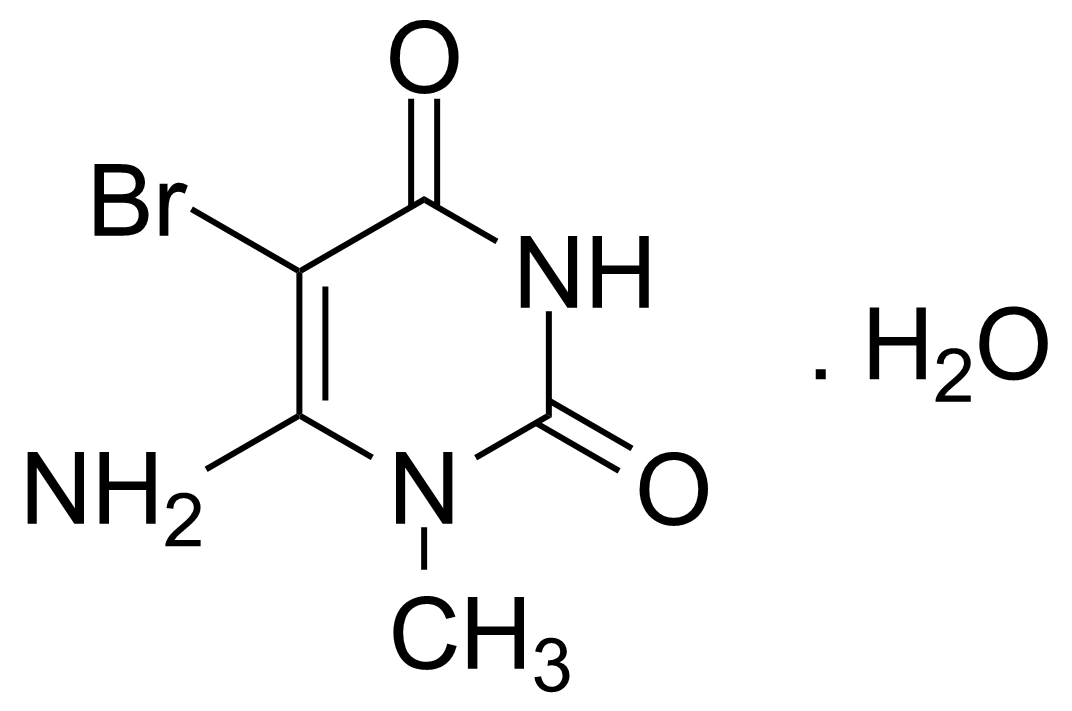 | [14094-37-2] | GEO-00087 |
| Nouveau | 2-Amino-5-chlorobenzonitrile | 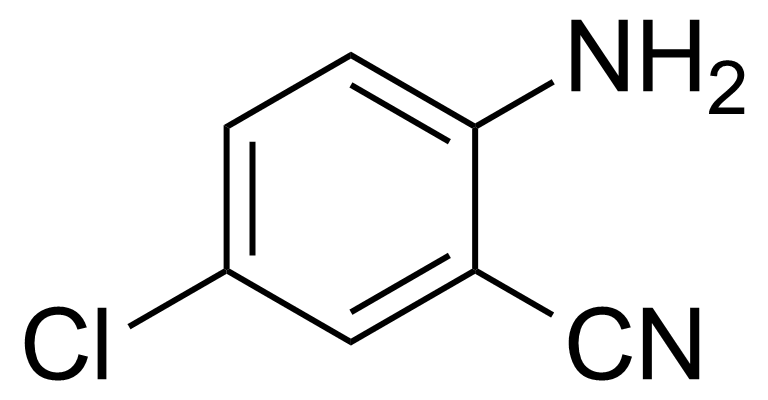 | [5922-60-1] | GEO-00097 |
| Nouveau | 2-Amino-4-chlorobenzothiazole | 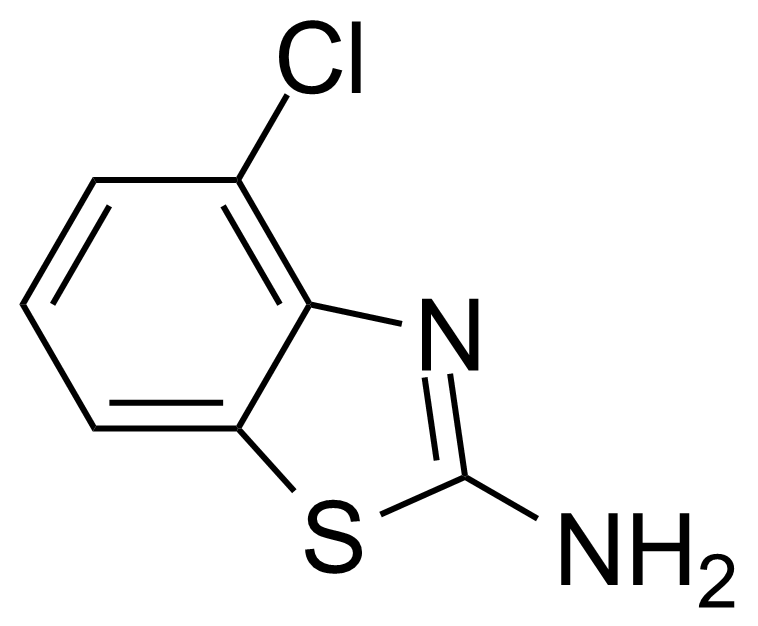 | [19952-47-7] | GEO-00099 |