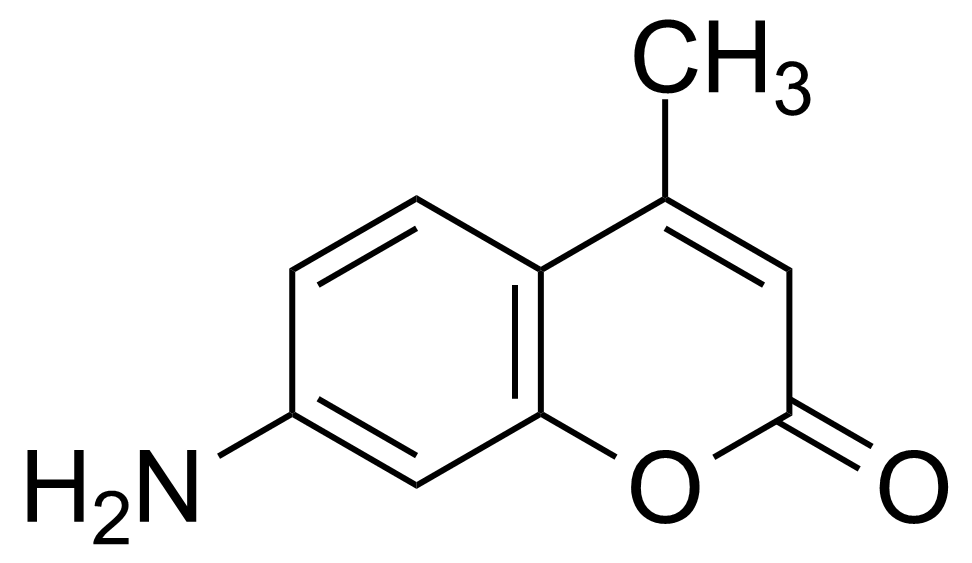
7-Amino-4-methylcoumarin
Ce produit n’est plus fabriqué, mais il nous reste encore un peu de stock.
Numéro CAS[26093-31-2]
G-codeGEO-02697
Formule moléculaireC10H9NO2
Poids moléculaire175.19
Synonymes
Coumarin 120 ; 7-Amino-4-methyl-2H-chromen-2-one
Pour plus d’informations ou si vous avez des questions, veuillez nous envoyer un e-mail georganics@georganics.sk ou utiliser notre formulaire de contact
Informations réglementaires
Ce produit n’a pas été classé.
Catégorisation des produits
Catégorie principale
Deuxième niveau
Troisième niveau
Description
7-Amino-4-methylcoumarin est un composé chimique utile avec une variété d'utilisations de recherche. Nous sommes heureux d'offrir des 7-Amino-4-methylcoumarin de haute qualité dans différentes tailles (pour la recherche, l’échelle pilote ou les applications de production) du milligramme aux lots de plusieurs kilogrammes, ce qui vous permet de sélectionner facilement la bonne quantité pour vos besoins.
Afficher la description complèteUnfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description of 7-Amino-4-methylcoumarin:
7-Amino-4-methylcoumarin [26093-31-2] (AMC), also known as Coumarin 120 belongs to the family of benzo-α-pyrones, called coumarins. It is a yellow crystalline solid with the melting point of 222-223 °C. It is soluble in common organic solvents.[1] 7-amino-4-methylcoumarin laser dye can emit laser in the range of 370-760 nm with the maximum λ at 354 nm (in ethanol). This compound is sensitive to the light and oxidizing agents therefore should be store in a dark, dry, tightly closed container. The most common method of preparation was published by Pechmann and Schwarz in 1899. It is based on the condensation of m-aminophenol with acetoacetic ester by heating in an alcohol in the presence of zinc chloride. Using this procedure, the AMC can be obtained in one step.[2]Application of 7-Amino-4-methylcoumarin:
Coumarin 120 is widely used as commercially available building block in the synthesis of fluorescent probes for a variety of sensing experiments in the enzymology. Such π-π conjugated system with electron-rich and charge transfer properties leads to the applications as fluorescent sensors for biological activities. Traditionally, coumarin substrates have been used to measure oxidative activities of cytochrome P450 (CYP) enzymes.[3] Moreover, it is widely used for peptide labeling in the study of proteases.[4] Coumarin-based chemosenors are used in selective detection of trace metals.[5] 7-Amino-4-methylcoumarin shows potential as antitubercular agent (the lowest MIC of 1 mg/L) by cell-wall-attacking mechanism of action.[6]Product categorization (Chemical groups):
Main category: Second level: Third level: ______________________________________________________________________________________[1] M. Zimmerman, E. Yurewicz, G. Patel Anal. Biochem. 1976, 70, 258. [2] H. Pechmann, O. Schwarz, Ber. Dtsch. Chem. Ges. 1899, 32, 3696. [3] D. Kim, Z. Wu, F. P. Guengerich J. Cell Biol. 2005, 280, 40319. [4] A. F. Kisselev, A. L. Goldberg Meth. Enzymol. 2005, 398, 364.
Kanaoka, T. Takahashi, H. Nakayama, T. Ueno, T. Sekine Chem. Pharm. Bull. 1982, 30, 1485.
Ishida, Y. Nakamura, T. Ohta, Y. Oe Molecules 2021, 26, 482. [5] L. Huang, W. Sheng, L. Wang, X. Meng, H. Duan, L. Chi RSC Adv, 2021, 11, 23597.
Muthusamy, K. Rajalakshmi, D. Zhu, W. Zhu, S. Wang, K. Lee, H. Xu, L. Zhao Sens. Actuators B Chem. 2021, 346, 130534. [6] R. Tandon, P. Ponnan, N. Aggarwal, R. Pathak, A. S. Baghel, G. Gupta, A. Arya, M. Nath, V. S. Parmar, H. G. Raj, A. K. Prasad, M. Bose, J. Antimicrob. Chemother. 2011, 66, 2543.
Produits similaires
| Nom du produit | Structure | Numéro CAS | G-code | |
|---|---|---|---|---|
| Nouveau | 5-Acetyl-2-amino-4-(2-furanyl)-6-methyl-4H-pyran-3-carbonitrile | 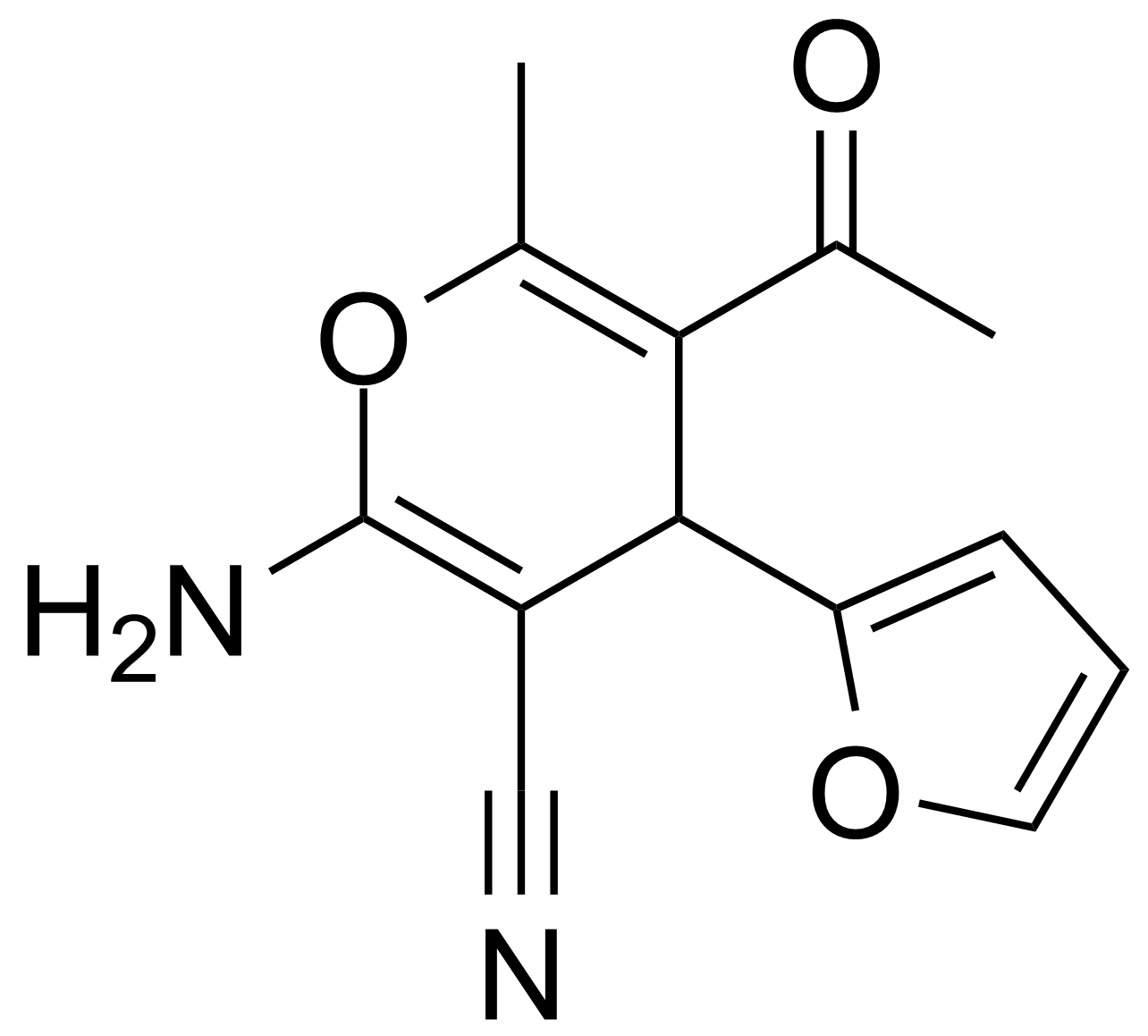 | [105263-08-9] | GEO-00016 |
| Nouveau | 5-Acetyl-2-amino-4-(4-methoxyphenyl)-6-methyl-4H-pyran-3-carbonitrile | 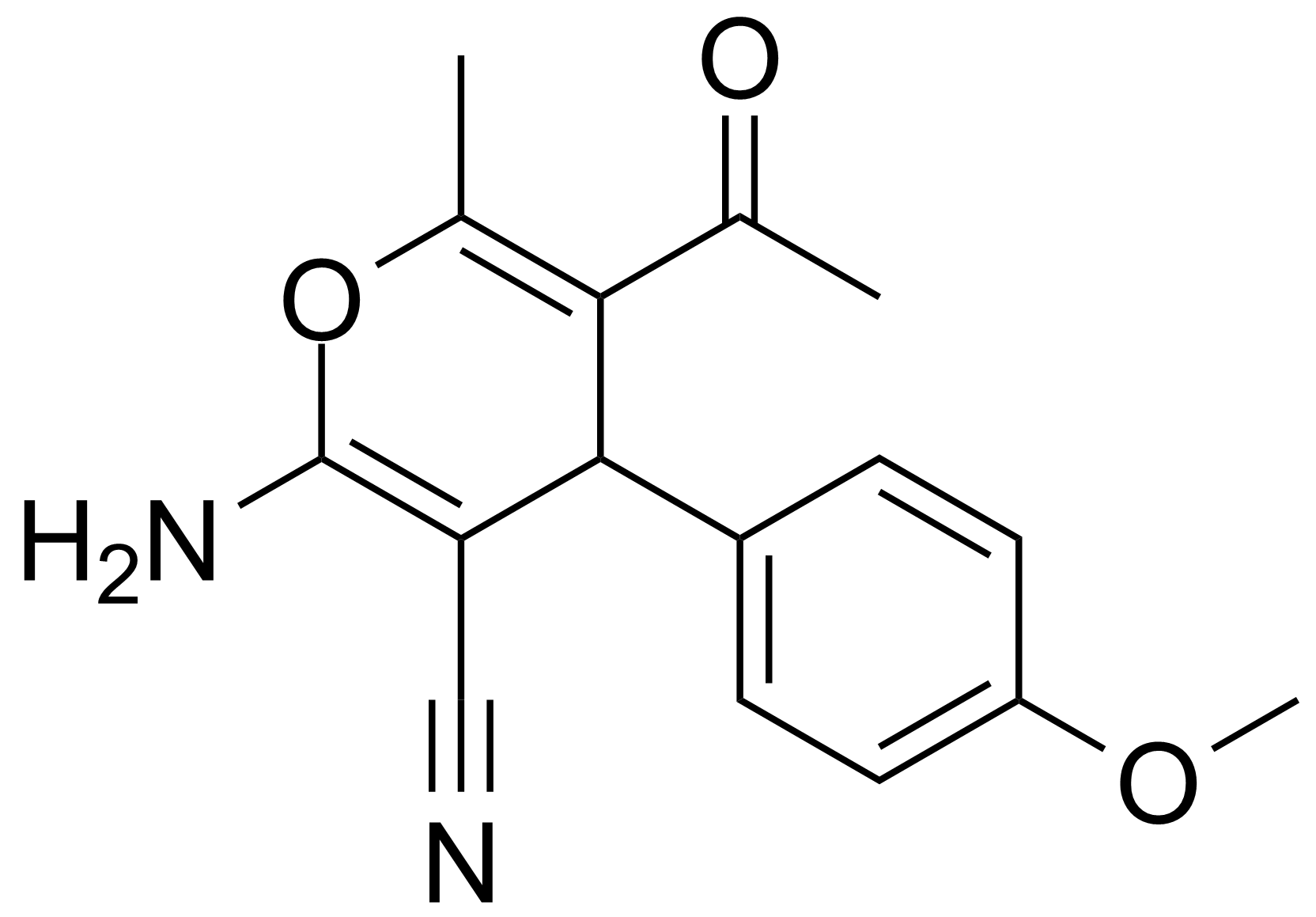 | [105263-07-8] | GEO-00017 |
| 5-Acetyl-2-amino-6-methyl-4-phenyl-4H-pyran-3-carbonitrile | 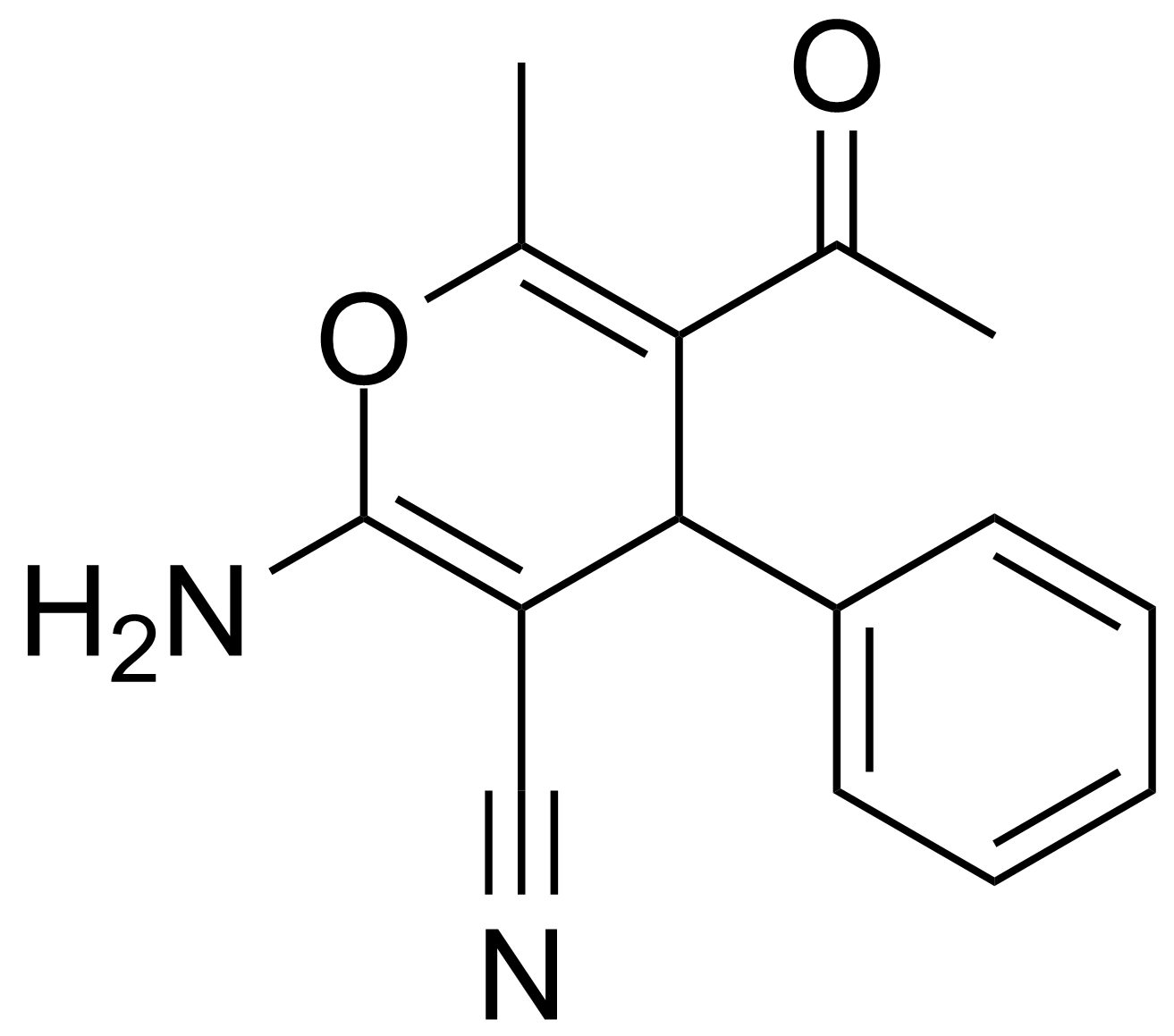 | [89809-89-2] | GEO-00018 | |
| Nouveau | 2-Acetylaminothiazole | 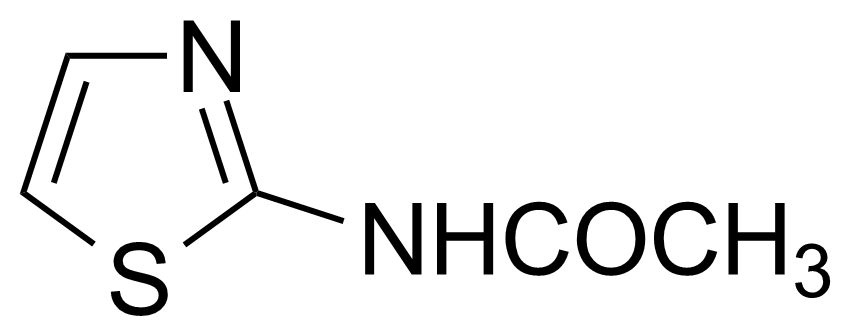 | [2719-23-5] | GEO-00020 |
| Nouveau | Allylcyclohexylamine | 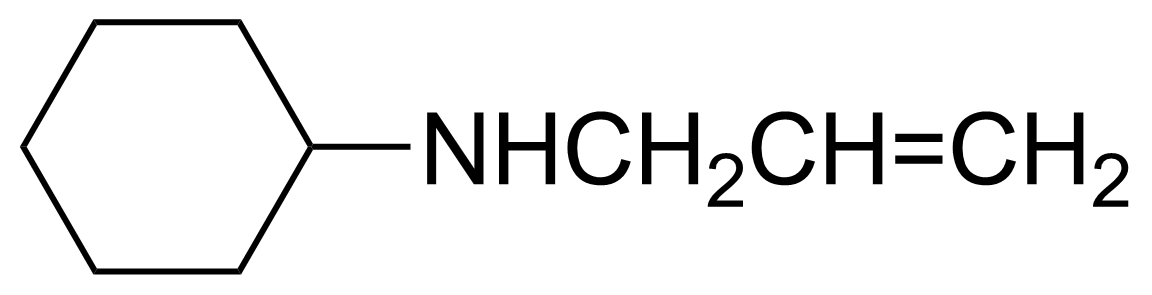 | [6628-00-8] | GEO-00059 |
| Nouveau | 4-Amino-2,1,3-benzothiadiazole | 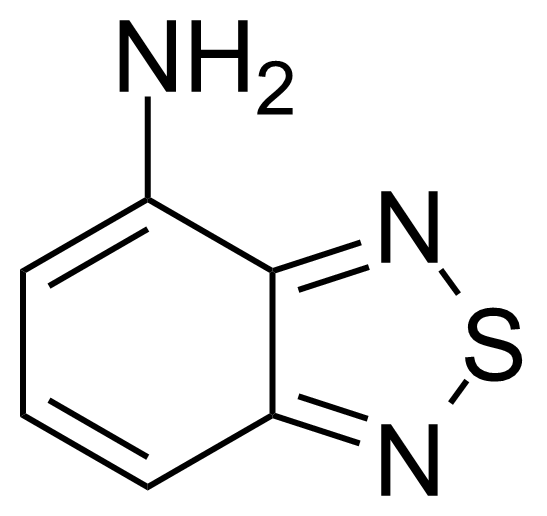 | [767-64-6] | GEO-00070 |
| Nouveau | 2-Aminobenzothiazole | 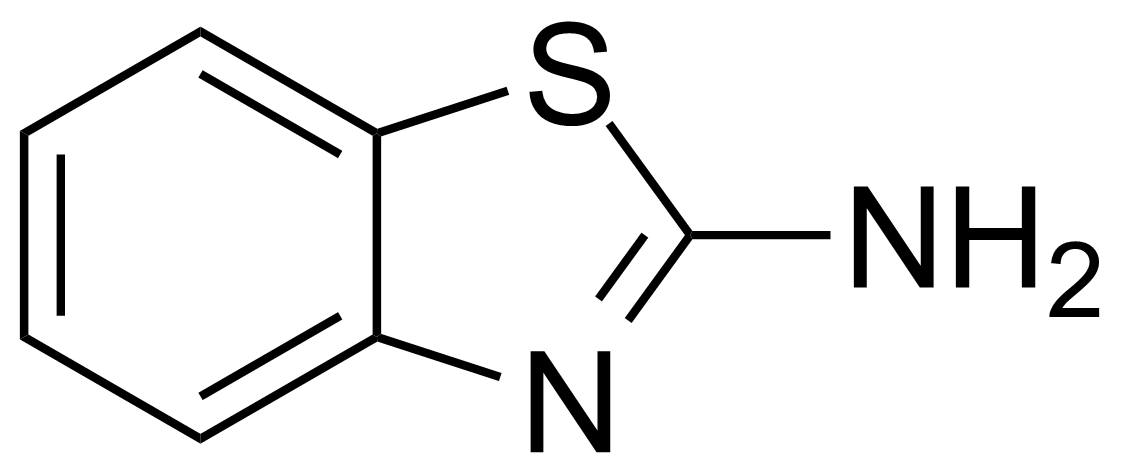 | [136-95-8] | GEO-00068 |
| Nouveau | 5-Amino-2-bromobenzoic acid | 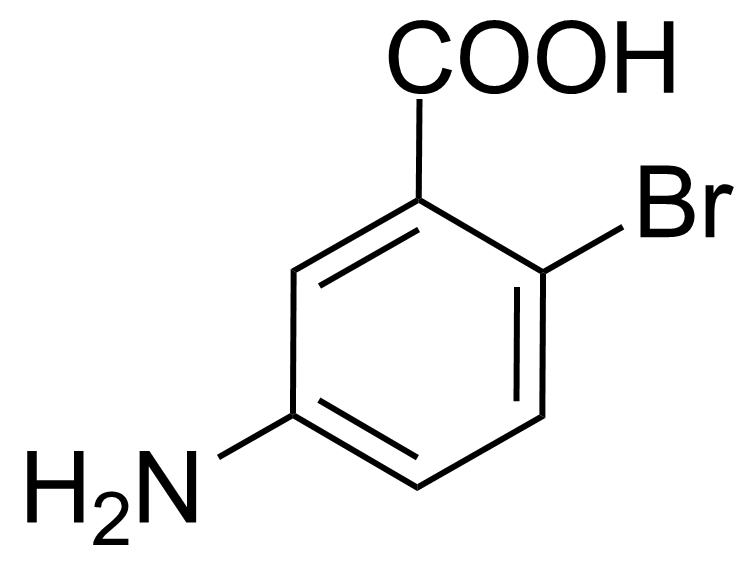 | [2840-02-0] | GEO-00082 |
| Nouveau | 2-Amino-6-bromobenzothiazole | 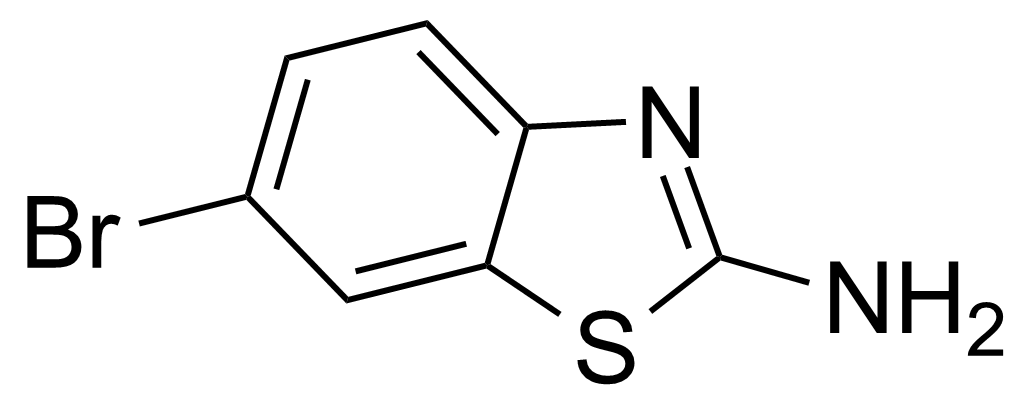 | [15864-32-1] | GEO-00083 |
| Nouveau | 6-Amino-5-bromo-1-methyluracil monohydrate | 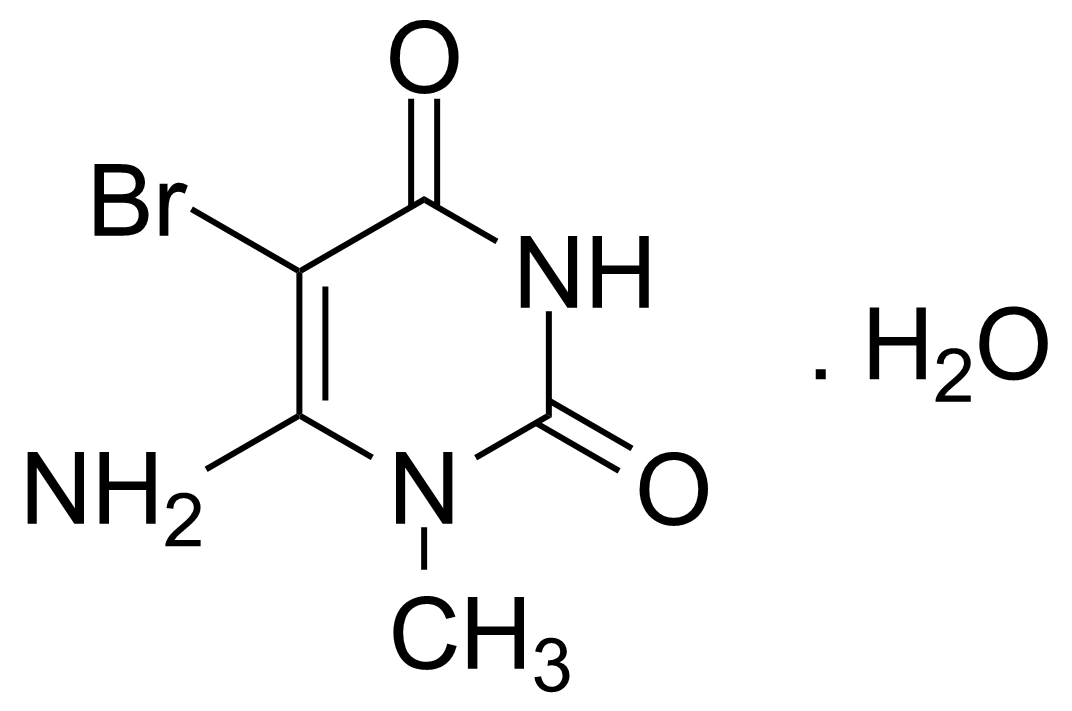 | [14094-37-2] | GEO-00087 |