 Janvier 01, 1970
Janvier 01, 1970Maleimide – general description and preparation
Unfortunately, this article is currently only in English language. We are working on a translation. Thank you for understanding.
General description and preparation of Maleimide:
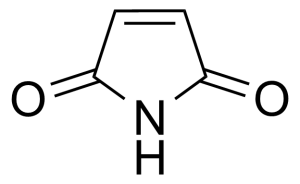
Maleimide [541-59-3] or 2,5-Pyrroledione is a unsaturated imide which name is a contraction of maleic acid and imide. It is a white crystalline solid with the melting point of 92-93 °C.[1] Maleimide can be synthesized via thermal decomposition of N-carbamoylmaleimide formed by reaction of maleic anhydride with urea.[2] In general, maleimides are produced by ring-closure imidation of various maleinamic acids in an organic solvent capable of forming an azeotrope with water in the presence of an acid catalyst and metal-containing compound (zinc acetate) as a promoter.[3]
Application of Maleimide:
Maleimide is versatile building block in organic synthesis. A special feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by Michael additions or via Diels-Alder reactions. Natural maleimide derivatives (ferinomalein, showdomycin, pencolide, turrapubesin) with the promising biological activities were isolated from bacteria and fungi.[4] Recently, maleimide, N-ethylmaleimide, N-methylmaleimide and N-phenylmaleimide have attracted the interest due to the cytotoxicity toward tumor cell lines through the inhibition of human topoisomerase II.[5] Maleimide-mediated methodologies are among the most used in bioconjugation.[6] Maleimide-functionalised polymers and liposomes exhibit enhanced ability to adhere to mucosal surfaces (mucoadhesion) due to the reactions with thiol-containing mucins.[7]
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
_______________________________________________________________________
[2] P. O. Tawney, R. H. Snyder, C. E. Bryan, R. P. Cogner, F. S. Dovell, R. J. Kelly, C. H. Stiteler J. Org. Chem. 1960, 25 (1), 56. doi:10.1021/jo01071a017
[3] Y. Kita, K. Sakamoto, M. Baba, A. Okubo Method for production of maleimides 1985, Nippon Shokubai Co Ltd. EP0165574A2.
[4] S. P. Putri, H. Kinoshita, F. Ihara, Y. Igarashi, T. Nihira J. Nat. Prod. 2009, 72, 1544. doi:10.1021/np9002806
[5] L. H. Jensen, A. Rondon-Corniere, I. Wessel, S. W. Langer, B. Søkilde, E. V. Carstensen, M. Sehested, P. B. Jensen Mol. Pharmacol. 2002, 61 (5), 1235. doi:10.1124/mol.61.5.1235
[6] O. Koniev, A. Wagner Chem. Soc. Rev. 2015, 44, 5495. doi:10.1039/C5CS00048C
[7] D. B. Kaldybekov, P. Tonglairoum, P. Opanasopit, V. V. Khutoryanskiy Eur. J. Pharm. Sci. 2018, 111, 83. doi:10.1016/j.ejps.2017.09.039