 Janvier 01, 1970
Janvier 01, 1970Methyl 2-amino-3-thiophenecarboxylate – description and application
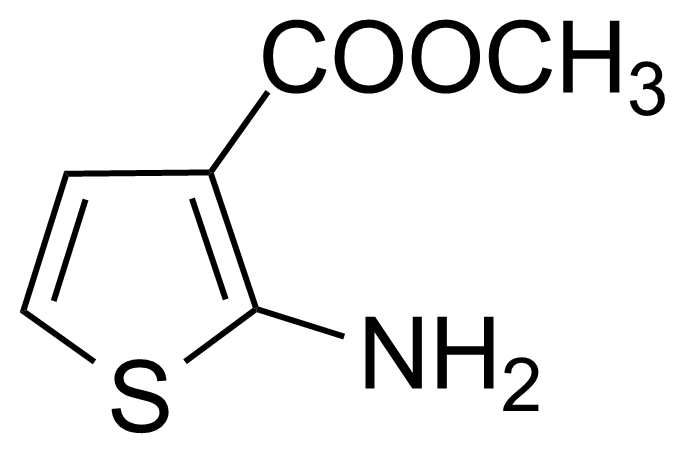
Methyl 2-amino-3-thiophenecarboxylate [4651-81-4] or 2-amino-3-carbomethoxythiophene or 2-amino-3-thiophenecarboxylic acid methylester is a white solid with the melting point of 72-73 °C.[1]
Preparation of Methyl 2-amino-3-thiophenecarboxylate:
It can be prepared by modifed Gewald reaction[2] using methyl cyanoacetate and 1,4-dithiane-2,5-diol in the presence of triethylamine in methanol. [3]
Application of Methyl 2-amino-3-thiophenecarboxylate:
2-Aminothiophene derivatives are important five-membered building blocks in organic synthesis. Urea derivatives of 2-amino-3-carbomethoxythiophene exhibited promising in vitro cytotoxicity against a human cancer cell lines as an effective binders of ribonucleotidereductase protein.[4] It was used as a building block in synthesis of various thienopyrimidine derivatives which are interesting structural element in development of pharmaceutical compounds.[5] Among others, these heterocycles have been used as part of kinase inhibitors to regulate dysfunctional cell signalling in cancer cells,[6] as calcium receptor antagonists,[7] transglutaminase inhibitors,[8] as peptidase IV inhibitors[9] and against hepatitis C virus infections.[10]
Product categorization (Chemical groups):
Main category:
Second level:
Third level:
_______________________________________________________________________
[2] K. Gewald, E. Schinke, H. Böttcher Chem. Ber. 1966, 99 (1), 94. doi:10.1002/cber.19660990116
[3] J. E. Hempel, A. G. Cadar, C. C. Hong Bioorg. Med. Chem. Lett. 2016, 26 (8), 1947. doi:10.1016/j.bmcl.2016.03.013
[4] V. Vikram, S. R. Renumutchu, R. Vankayala, S. Thangudu, K. Rao, A. Parimi, U. Parimi J. Chem. Sci. 2020, 132, 126. doi:10.1007/s12039-020-01834-w
[5][5] V. P. Litvinov Adv. Heterocycl. Chem. 2006, 92, 83. doi:10.1016/S0065-2725(06)92003-0
[6] S. J. Baker, P. J. Goldsmith, T. C. Hancox, N. A. Pegg, S. Price, S. J. Shuttleworth, S. Sohal Pyrimidine derivatives as pi3k inhibitors 2006, F.Hoffmann-La Roche Ag WO2007122410A1.
[7] F. Bi, T. Didiuk, A. Guzman-Perez, D. A. Griffith, K. K. C. Liu, D. P. Walker, M. P. Zawistoski Thieno[2,3-d]pyrimidin-4(3h)-one, isoxazolo[5,4-d]pyrimidin-4(5h)-one and isothiazolo[5,4-d]pyrimidin-4(5h)-one derivatives as calcium receptor antagonists 2008, Pfizer Products Inc. WO2009001214A2.
[8] R. L. Stein, A. Case, L. A. Yeh, G. Cuny, E. Duval Substitued 3,4-dihydrothieno [2,3-d] pyrmidines as tissue transglutaminase inhibitors 2005, The Brigham And Women’s Hospital, Inc. WO2006060702A1.
[9] J. Deng, L. Peng, G. Zhang, X. Lan, C. Li, F. Chen, Y. Zhou, Z. Lin, L. Chen, R. Dai, H. Xu, L. Yang, X. Zhang and W. Hu Eur. J. Med. Chem. 2010, 46, 71.
[10] C. A. Broka, R. T. Hendricks, H. Maag, D. B. Smith, J. Wanner Heterocyclic antiviral compounds 2011, Roche Palo Alto LLC 20110070190.